CCNA2 Ablation in Aged Mice Results in Abnormal rRNA Granule Accumulation in Hippocampus
- PMID: 30579783
- PMCID: PMC6412465
- DOI: 10.1016/j.ajpath.2018.10.020
CCNA2 Ablation in Aged Mice Results in Abnormal rRNA Granule Accumulation in Hippocampus
Abstract
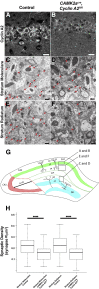
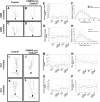
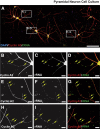
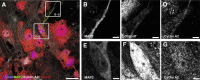
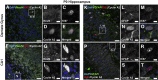
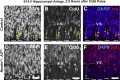
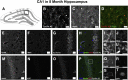
Mounting evidence in the literature suggests that RNA-RNA binding protein aggregations can disturb neuronal homeostasis and lead to symptoms associated with normal aging as well as dementia. The specific ablation of cyclin A2 in adult neurons results in neuronal polyribosome aggregations and learning and memory deficits. Detailed histologic and ultrastructural assays of aged mice revealed that post-mitotic hippocampal pyramidal neurons maintain cyclin A2 expression and that proliferative cells in the dentate subgranular zone express cyclin A2. Cyclin A2 loss early during neural development inhibited hippocampal development through canonical/cell-cycle mechanisms, including prolonged cell cycle timing in embryonic hippocampal progenitor cells. However, in mature neurons, cyclin A2 colocalized with dendritic rRNA. Cyclin A2 ablation in adult hippocampus resulted in decreased synaptic density in the hippocampus as well as in accumulation of rRNA granules in dendrite shafts. We conclude that cyclin A2 functions in a noncanonical/non-cell cycle regulatory role to maintain adult pyramidal neuron ribostasis.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Garcia-Moreno L.M., Conejo N.M., Pardo H.G., Gomez M., Martin F.R., Alonso M.J., Arias J.L. Hippocampal AgNOR activity after chronic alcohol consumption and alcohol deprivation in rats. Physiol Behav. 2001;72:115–121. - PubMed
-
- Kiryk A., Sowodniok K., Kreiner G., Rodriguez-Parkitna J., Sonmez A., Gorkiewicz T., Bierhoff H., Wawrzyniak M., Janusz A.K., Liss B., Konopka W., Schutz G., Kaczmarek L., Parlato R. Impaired rRNA synthesis triggers homeostatic responses in hippocampal neurons. Front Cell Neurosci. 2013;7:207. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

