Lipoproteins as targets and markers of lipoxidation
- PMID: 30579928
- PMCID: PMC6859580
- DOI: 10.1016/j.redox.2018.101066
Lipoproteins as targets and markers of lipoxidation
Abstract
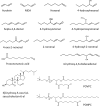
Lipoproteins are essential systemic lipid transport particles, composed of apolipoproteins embedded in a phospholipid and cholesterol monolayer surrounding a cargo of diverse lipid species. Many of the lipids present are susceptible to oxidative damage by lipid peroxidation, giving rise to the formation of reactive lipid peroxidation products (rLPPs). In view of the close proximity of the protein and lipid moieties within lipoproteins, the probability of adduct formation between rLPPs and amino acid residues of the proteins, a process called lipoxidation, is high. There has been interest for many years in the biological effects of such modifications, but the field has been limited to some extent by the availability of methods to determine the sites and exact nature of such modification. More recently, the availability of a wide range of antibodies to lipoxidation products, as well as advances in analytical techniques such as liquid chromatography tandem mass spectrometry (LC-MSMS), have increased our knowledge substantially. While most work has focused on LDL, oxidation of which has long been associated with pro-inflammatory responses and atherosclerosis, some studies on HDL, VLDL and Lipoprotein(a) have also been reported. As the broader topic of LDL oxidation has been reviewed previously, this review focuses on lipoxidative modifications of lipoproteins, from the historical background through to recent advances in the field. We consider the main methods of analysis for detecting rLPP adducts on apolipoproteins, including their advantages and disadvantages, as well as the biological effects of lipoxidized lipoproteins and their potential roles in diseases.
Keywords: ApoB-100; Atherosclerosis; HDL; Immunoassays; LDL; Lipid peroxidation; Liquid chromatography mass spectrometry.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





References
-
- K.R. Feingold, C. Grunfeld, Introduction to Lipids and Lipoproteins, in: A.V. Leslie, J. De Groot, Editor-in-chief, George Chrousos, Kathleen Dungan, Kenneth R. Feingold, Ashley Grossman, Jerome M. Hershman, Christian Koch, Márta Korbonits, Robert McLachlan, Maria New, Jonathan Purnell, Robert Rebar, Frederick Singer (Ed.), Endotext, South Dartmouth (MA): MDText.com, Inc., 2000.
-
- Steinbrecher U.P., Witztum J.L., Parthasarathy S., Steinberg D. Decrease in reactive amino groups during oxidation or endothelial cell modification of LDL. Correlation with changes in receptor-mediated catabolism. Arteriosclerosis. 1987 - PubMed
-
- Parthasarathy S., Steinberg D., Witztum J.L. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu. Rev. Med. 1992 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

