Diallyl disulfide inhibits ethanol-induced pulmonary cell vitamin D and antimicrobial peptide cathelicidin depletion
- PMID: 30580017
- PMCID: PMC7194279
- DOI: 10.1016/j.alcohol.2018.12.003
Diallyl disulfide inhibits ethanol-induced pulmonary cell vitamin D and antimicrobial peptide cathelicidin depletion
Abstract
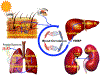
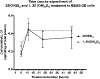
Ethanol has been found to affect pulmonary cells by interfering with vitamin D metabolism and pulmonary defense mechanisms. The objective of this study was to understand the mechanisms of ethanol's disruptive influence on the vitamin D pathway and inhibition of anti-microbial peptide cathelicidin (LL-37). Bronchial epithelial cells (BEAS-2Bs), primary human bronchial epithelial cells (HBECs), primary human alveolar epithelial cells (HPAEpiCs), and human monocyte cells (THP-1s) were used in this study. These cells were cultured and exposed to different treatment groups: medium-only control, ethanol (70 mM) only, diallyl disulfide (DADS) (10 μM) -only, and a co-exposure of ethanol (70 mM) and DADS (10 μM) for 10 or 24 h. Calcidiol (50 ng/mL) and calcitriol (0.05 ng/mL) dose-response studies were conducted for 48 h. After incubation, cells were trypsinized, lysed, and centrifuged, and the cellular lysate was prepared for assay. Protein was quantified, and levels of inactive vitamin D [25(OH)D3], active vitamin D [1, 25(OH)2 D3], and anti-microbial peptides (cathelicidin/LL-37) in the samples were assayed using commercially available ELISA kits. In the ethanol-exposed group, cellular lysate concentrations of 25(OH)D3 and LL-37 were significantly reduced by 30%, and 40% in BEAS-2B cells, and 35% and 80% in HPAEpi cells respectively. Overall 1, 25(OH)2D3 cellular lysate levels were lower but followed a similar trend as the 25(OH)D3 response. LL-37 levels in primary bronchial, alveolar cells, and ThP-1 cells were statistically reduced in ethanol-exposed groups (60%, 80%, and 65%, respectively) when compared with control. Following the addition of DADS, levels of LL-37 were recovered to within control levels for all three cell types. This study establishes two clinically relevant observations: that the exposure of pulmonary epithelial and monocyte cells to physiologically relevant levels of excessive ethanol selectively disrupts the activation of pulmonary vitamin D and inhibits the presence of anti-microbial peptide (LL-37) in vitro, and the co-exposure of DADS significantly attenuates ethanol-induced intracellular LL-37 depletion.
Keywords: Active vitamin D; Alcohol; Alcohol use disorder (AUD); Anti-microbial peptides; Cathelicidin/LL-37; Inactive vitamin D; Pneumonia; Pulmonary system.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
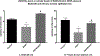

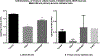



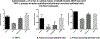

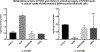
References
-
- Bouillon R, De Groot LJ, & Jameson JL (2001). Vitamin D: From photosynthesis, metabolism, and action to clinical applications. Saunders; Retrieved from https://clinicalgate.com/vitamin-d-from-photosynthesis-metabolism-and-ac...
-
- Cesur Y, Caksen H, Gündem A, Kirimi E, & Odabaş D (2003). Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. Journal of Pediatric Endocrinology & Metabolism, 16, 1105–1109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

