Overexpression of plasmepsin II and plasmepsin III does not directly cause reduction in Plasmodium falciparum sensitivity to artesunate, chloroquine and piperaquine
- PMID: 30580023
- PMCID: PMC6304341
- DOI: 10.1016/j.ijpddr.2018.11.004
Overexpression of plasmepsin II and plasmepsin III does not directly cause reduction in Plasmodium falciparum sensitivity to artesunate, chloroquine and piperaquine
Abstract
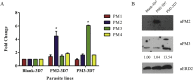
Artemisinin derivatives and their partner drugs in artemisinin combination therapies (ACTs) have played a pivotal role in global malaria mortality reduction during the last two decades. The loss of artemisinin efficacy due to evolving drug-resistant parasites could become a serious global health threat. Dihydroartemisinin-piperaquine is a well tolerated and generally highly effective ACT. The implementation of a partner drug in ACTs is critical in the control of emerging artemisinin resistance. Even though artemisinin is highly effective in parasite clearance, it is labile in the human body. A partner drug is necessary for killing the remaining parasites when the pulses of artemisinin have ceased. A population of Plasmodium falciparum parasites in Cambodia and adjacent countries has become resistant to piperaquine. Increased copy number of the genes encoding the haemoglobinases Plasmepsin II and Plasmepsin III has been linked with piperaquine resistance by genome-wide association studies and in clinical trials, leading to the use of increased plasmepsin II/plasmepsin III copy number as a molecular marker for piperaquine resistance. Here we demonstrate that overexpression of plasmepsin II and plasmepsin III in the 3D7 genetic background failed to change the susceptibility of P. falciparum to artemisinin, chloroquine and piperaquine by both a standard dose-response analysis and a piperaquine survival assay. Whilst plasmepsin copy number polymorphism is currently implemented as a molecular surveillance resistance marker, further studies to discover the molecular basis of piperaquine resistance and potential epistatic interactions are needed.
Keywords: Artemisinin; Drug resistance; Malaria; Piperaquine; Plasmepsin.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
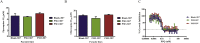

Similar articles
-
Are k13 and plasmepsin II genes, involved in Plasmodium falciparum resistance to artemisinin derivatives and piperaquine in Southeast Asia, reliable to monitor resistance surveillance in Africa?Malar J. 2019 Aug 23;18(1):285. doi: 10.1186/s12936-019-2916-6. Malar J. 2019. PMID: 31443646 Free PMC article.
-
The plasmepsin-piperaquine paradox persists in Plasmodium falciparum.PLoS Pathog. 2025 Jul 28;21(7):e1012779. doi: 10.1371/journal.ppat.1012779. eCollection 2025 Jul. PLoS Pathog. 2025. PMID: 40720544 Free PMC article.
-
Association of a Novel Mutation in the Plasmodium falciparum Chloroquine Resistance Transporter With Decreased Piperaquine Sensitivity.J Infect Dis. 2017 Aug 15;216(4):468-476. doi: 10.1093/infdis/jix334. J Infect Dis. 2017. PMID: 28931241 Free PMC article.
-
Plasmodium falciparum Resistance to Artemisinin Derivatives and Piperaquine: A Major Challenge for Malaria Elimination in Cambodia.Am J Trop Med Hyg. 2016 Dec 7;95(6):1228-1238. doi: 10.4269/ajtmh.16-0234. Epub 2016 Oct 17. Am J Trop Med Hyg. 2016. PMID: 27928074 Free PMC article. Review.
-
Exploration of copy number variation in genes related to anti-malarial drug resistance in Plasmodium falciparum.Gene. 2020 Apr 30;736:144414. doi: 10.1016/j.gene.2020.144414. Epub 2020 Jan 30. Gene. 2020. PMID: 32006594 Review.
Cited by
-
Molecular Mechanisms of Drug Resistance in Plasmodium falciparum Malaria.Annu Rev Microbiol. 2020 Sep 8;74:431-454. doi: 10.1146/annurev-micro-020518-115546. Annu Rev Microbiol. 2020. PMID: 32905757 Free PMC article. Review.
-
How has mass drug administration with dihydroartemisinin-piperaquine impacted molecular markers of drug resistance? A systematic review.Malar J. 2022 Jun 11;21(1):186. doi: 10.1186/s12936-022-04181-y. Malar J. 2022. PMID: 35690758 Free PMC article.
-
Are k13 and plasmepsin II genes, involved in Plasmodium falciparum resistance to artemisinin derivatives and piperaquine in Southeast Asia, reliable to monitor resistance surveillance in Africa?Malar J. 2019 Aug 23;18(1):285. doi: 10.1186/s12936-019-2916-6. Malar J. 2019. PMID: 31443646 Free PMC article.
-
Elucidating Mechanisms of Drug-Resistant Plasmodium falciparum.Cell Host Microbe. 2019 Jul 10;26(1):35-47. doi: 10.1016/j.chom.2019.06.001. Cell Host Microbe. 2019. PMID: 31295423 Free PMC article. Review.
-
Development of copy number assays for detection and surveillance of piperaquine resistance associated plasmepsin 2/3 copy number variation in Plasmodium falciparum.Malar J. 2020 May 13;19(1):181. doi: 10.1186/s12936-020-03249-x. Malar J. 2020. PMID: 32404110 Free PMC article.
References
-
- Agrawal S., Moser K.A., Morton L., Cummings M.P., Parihar A., Dwivedi A., Shetty A.C., Drabek E.F., Jacob C.G., Henrich P.P., Parobek C.M., Jongsakul K., Huy R., Spring M.D., Lanteri C.A., Chaorattanakawee S., Lon C., Fukuda M.M., Saunders D.L., Fidock D.A., Lin J.T., Juliano J.J., Plowe C.V., Silva J.C., Takala-Harrison S. Association of a novel mutation in the Plasmodium falciparum chloroquine resistance transporter with decreased piperaquine sensitivity. J. Infect. Dis. 2017;216:468–476. - PMC - PubMed
-
- Amaratunga C., Lim P., Suon S., Sreng S., Mao S., Sopha C., Sam B., Dek D., Try V., Amato R., Blessborn D., Song L., Tullo G.S., Fay M.P., Anderson J.M., Tarning J., Fairhurst R.M. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect. Dis. 2016;16:357–365. - PMC - PubMed
-
- Amato R., Lim P., Miotto O., Amaratunga C., Dek D., Pearson R.D., Almagro-Garcia J., Neal A.T., Sreng S., Suon S., Drury E., Jyothi D., Stalker J., Kwiatkowski D.P., Fairhurst R.M. Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect. Dis. 2017;17:164–173. - PMC - PubMed
-
- Amato R., Pearson R.D., Almagro-Garcia J., Amaratunga C., Lim P., Suon S., Sreng S., Drury E., Stalker J., Miotto O., Fairhurst R.M., Kwiatkowski D.P. Origins of the current outbreak of multidrug-resistant malaria in southeast Asia: a retrospective genetic study. Lancet Infect. Dis. 2018;18:337–345. - PMC - PubMed
-
- Ashley E.A., Dhorda M., Fairhurst R.M., Amaratunga C., Lim P., Suon S., Sreng S., Anderson J.M., Mao S., Sam B., Sopha C., Chuor C.M., Nguon C., Sovannaroth S., Pukrittayakamee S., Jittamala P., Chotivanich K., Chutasmit K., Suchatsoonthorn C., Runcharoen R., Hien T.T., Thuy-Nhien N.T., Thanh N.V., Phu N.H., Htut Y., Han K.T., Aye K.H., Mokuolu O.A., Olaosebikan R.R., Folaranmi O.O., Mayxay M., Khanthavong M., Hongvanthong B., Newton P.N., Onyamboko M.A., Fanello C.I., Tshefu A.K., Mishra N., Valecha N., Phyo A.P., Nosten F., Yi P., Tripura R., Borrmann S., Bashraheil M., Peshu J., Faiz M.A., Ghose A., Hossain M.A., Samad R., Rahman M.R., Hasan M.M., Islam A., Miotto O., Amato R., MacInnis B., Stalker J., Kwiatkowski D.P., Bozdech Z., Jeeyapant A., Cheah P.Y., Sakulthaew T., Chalk J., Intharabut B., Silamut K., Lee S.J., Vihokhern B., Kunasol C., Imwong M., Tarning J., Taylor W.J., Yeung S., Woodrow C.J., Flegg J.A., Das D., Smith J., Venkatesan M., Plowe C.V., Stepniewska K., Guerin P.J., Dondorp A.M., Day N.P., White N.J., Tracking Resistance to Artemisinin C. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014;371:411–423. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

