Role of meprin metalloproteinases in cytokine processing and inflammation
- PMID: 30580156
- PMCID: PMC6414266
- DOI: 10.1016/j.cyto.2018.11.032
Role of meprin metalloproteinases in cytokine processing and inflammation
Abstract
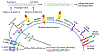
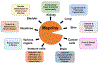
Meprin metalloendopeptidases, comprising α and β isoforms, are widely expressed in mammalian cells and organs including kidney, intestines, lungs, skin, and bladder, and in a variety of immune cells and cancer cells. Meprins proteolytically process many inflammatory mediators, including cytokines, chemokines, and other bioactive proteins and peptides that control the function of immune cells. The knowledge of meprin-mediated processing of inflammatory mediators and other target substrates provides a pathophysiologic link for the involvement of meprins in the pathogenesis of many inflammatory disorders. Meprins are now known to play important roles in inflammatory diseases including acute kidney injury, sepsis, urinary tract infections, bladder inflammation, and inflammatory bowel disease. The proteolysis of epithelial and endothelial barriers including cell junctional proteins by meprins promotes leukocyte influx into areas of tissue damage to result in inflammation. Meprins degrade extracellular matrix proteins; this ability of meprins is implicated in the cell migration of leukocytes and the invasion of tumor cells that express meprins. Proteolytic processing and maturation of procollagens provides evidence that meprins are involved in collagen maturation and deposition in the fibrotic processes involved in the formation of keloids and hypertrophic scars and lung fibrosis. This review highlights recent progress in understanding the role of meprins in inflammatory disorders in both human and mouse models.
Keywords: CCL2; IL-18; IL-1β; IL-6; IL-6R; Inflammation; Meprin A; Meprin α; Meprin β; Metalloproteinase.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


References
-
- Prox J, Arnold P, Becker-Pauly C, Meprin alpha and meprin beta: Procollagen proteinases in health and disease, Matrix Biol 44-46 (2015) 7–13. - PubMed
-
- Tang J, Bond JS, Maturation of secreted meprin alpha during biosynthesis: role of the furin site and identification of the COOH-terminal amino acids of the mouse kidney metalloprotease subunit, Arch Biochem Biophys 349(1) (1998) 192–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

