The impact of xanthine oxidase (XO) on hemolytic diseases
- PMID: 30580157
- PMCID: PMC6305892
- DOI: 10.1016/j.redox.2018.101072
The impact of xanthine oxidase (XO) on hemolytic diseases
Abstract
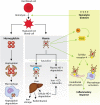
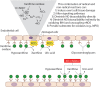
Hemolytic diseases are associated with elevated levels of circulating free heme that can mediate endothelial dysfunction directly via redox reactions with biomolecules or indirectly by upregulating enzymatic sources of reactive species. A key enzymatic source of these reactive species is the purine catabolizing enzyme, xanthine oxidase (XO) as the oxidation of hypoxanthine to xanthine and subsequent oxidation of xanthine to uric acid generates superoxide (O2•-) and hydrogen peroxide (H2O2). While XO has been studied for over 120 years, much remains unknown regarding specific mechanistic roles for this enzyme in pathologic processes. This gap in knowledge stems from several interrelated issues including: 1) lethality of global XO deletion and the absence of tissue-specific XO knockout models have coalesced to relegate proof-of-principle experimentation to pharmacology; 2) XO is mobile and thus when upregulated locally can be secreted into the circulation and impact distal vascular beds by high-affinity association to the glycocalyx on the endothelium; and 3) endothelial-bound XO is significantly resistant (> 50%) to inhibition by allopurinol, the principle compound used for XO inhibition in the clinic as well as the laboratory. While it is known that circulating XO is elevated in hemolytic diseases including sickle cell, malaria and sepsis, little is understood regarding its role in these pathologies. As such, the aim of this review is to define our current understanding regarding the effect of hemolysis (free heme) on circulating XO levels as well as the subsequent impact of XO-derived oxidants in hemolytic disease processes.
Keywords: Heme toxicity; Hemolysis; Reactive oxygen species; Therapeutics; Xanthine oxidase.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Vinchi F. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation. 2013;127(12):1317–1329. - PubMed
-
- Jain M.D., Cabrerizo-Sanchez R., Karkouti K., Yau T., Pendergrast J.M., Cserti-Gazdewich C.M. Seek and you shall find--but then what do you do? Cold agglutinins in cardiopulmonary bypass and a single-center experience with cold agglutinin screening before cardiac surgery. Transfus. Med. Rev. 2013;27(2):65–73. - PubMed
-
- Iwalokun B.A., Bamiro S.B., Ogunledun A. Levels and interactions of plasma xanthine oxidase, catalase and liver function parameters in Nigerian children with Plasmodium falciparum infection. APMIS. 2006;114(12):842–850. - PubMed
-
- Luchtemberg M.N. Xanthine oxidase activity in patients with sepsis. Clin. Biochem. 2008;41(14–15):1186–1190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

