Mimicking Microbial Rhodopsin Isomerization in a Single Crystal
- PMID: 30580520
- PMCID: PMC7358036
- DOI: 10.1021/jacs.8b12493
Mimicking Microbial Rhodopsin Isomerization in a Single Crystal
Abstract
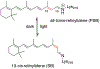
Bacteriorhodopsin represents the simplest, and possibly most abundant, phototropic system requiring only a retinal-bound transmembrane protein to convert photons of light to an energy-generating proton gradient. The creation and interrogation of a microbial rhodopsin mimic, based on an orthogonal protein system, would illuminate the design elements required to generate new photoactive proteins with novel function. We describe a microbial rhodopsin mimic, created using a small soluble protein as a template, that specifically photoisomerizes all- trans to 13- cis retinal followed by thermal relaxation to the all- trans isomer, mimicking the bacteriorhodopsin photocycle, in a single crystal. The key element for selective isomerization is a tuned steric interaction between the chromophore and protein, similar to that seen in the microbial rhodopsins. It is further demonstrated that a single mutation converts the system to a protein photoswitch without chromophore photoisomerization or conformational change.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
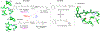
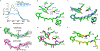

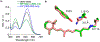
References
-
- Schotte F; Cho HS; Kaila VRI; Kamikubo H; Dashdorj N; Henry ER; Graber TJ; Henning R; Wulff M; Hummer G; Kataoka M; Anfinrud PA Watching a signaling protein function in real time via 100-ps time-resolved Laue crystallography. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 19256. - PMC - PubMed
- Ernst OP; Lodowski DT; Elstner M; Hegemann P; Brown LS; Kandori H Microbial and Animal Rhodopsins: Structures, Functions, and Molecular Mechanisms. Chem. Rev 2014, 114, 126. - PMC - PubMed
- Sudo Y; Ihara K; Kobayashi S; Suzuki D; Irieda H; Kikukawa T; Kandori H; Homma M A microbial rhodopsin with a unique retinal composition shows both sensory rhodopsin II and bacteri<EDorhodopsin-like properties. J. Biol. Chem 2011, 286, 5967. - PMC - PubMed
-
- Béjà O; Aravind L; Koonin EV; Suzuki MT; Hadd A; Nguyen LP; Jovanovich SB; Gates CM; Feldman RA; Spudich JL; Spudich EN; DeLong EF Bacterial Rhodopsin: Evidence for a New Type of Phototrophy in the Sea. Science 2000, 289, 1902. - PubMed
-
- Gordeliy VI; Labahn J; Moukhametzianov R; Efremov R; Granzin J; Schlesinger R; Buldt G; Savopol T; Scheidig AJ; Klare JP; Engelhard M Molecular basis of transmembrane signalling by sensory rhodopsin II-transducer complex. Nature 2002, 419, 484. - PubMed
-
- Govorunova EG; Koppel LA The Road to Optogenetics: Microbial Rhodopsins. Biochemistry 2016, 81, 928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

