A dual Ucp1 reporter mouse model for imaging and quantitation of brown and brite fat recruitment
- PMID: 30580967
- PMCID: PMC6358570
- DOI: 10.1016/j.molmet.2018.11.009
A dual Ucp1 reporter mouse model for imaging and quantitation of brown and brite fat recruitment
Abstract
Objectives: Brown adipose tissue (BAT) dissipates nutritional energy as heat through uncoupling protein 1 (UCP1). The discovery of functional BAT in healthy adult humans has promoted the search for pharmacological interventions to recruit and activate brown fat as a treatment of obesity and diabetes type II. These efforts require in vivo models to compare the efficacy of novel compounds in a relevant physiological context.
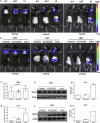
Methods: We generated a knock-in mouse line expressing firefly luciferase and near-infrared red florescent protein (iRFP713) driven by the regulatory elements of the endogenous Ucp1 gene.
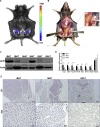
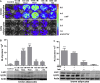
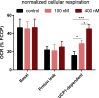
Results: Our detailed characterization revealed that firefly luciferase activity faithfully reports endogenous Ucp1 gene expression in response to physiological and pharmacological stimuli. The iRFP713 fluorescence signal was detected in the interscapular BAT region of cold-exposed reporter mice in an allele-dosage dependent manner. Using this reporter mouse model, we detected a higher browning capacity in female peri-ovarian white adipose tissue compared to male epididymal WAT, which we further corroborated by molecular and morphological features. In situ imaging detected a strong luciferase activity signal in a previously unappreciated adipose tissue depot adjunct to the femoral muscle, now adopted as femoral brown adipose tissue. In addition, screening cultured adipocytes by bioluminescence imaging identified the selective Salt-Inducible Kinase inhibitor, HG-9-91-01, to increase Ucp1 gene expression and mitochondrial respiration in brown and brite adipocytes.
Conclusions: In our mouse model, firefly luciferase activity serves as a bona fide reporter for dynamic regulation of Ucp1. In addition, by means of iRFP713 we are able to monitor Ucp1 expression in a non-invasive fashion.
Keywords: BAT; Browning; Firefly luciferase; Thermogenesis; UCP1; WAT; iRFP713.
Copyright © 2018. Published by Elsevier GmbH.
Figures








References
-
- Smorlesi A., Frontini A., Cinti S. Stem cells in aesthetic procedures: art, science, and clinical techniques. 2001. The adipose organ: morphological perspectives of adipose tissues; pp. 123–133.
-
- Klingenspor M., Bast A., Bolze F., Li Y., Maurer S., Schweizer S. Springer; Cham: 2017. Brown adipose tissue. Adipose tissue biology; pp. 91–147.
-
- Klingenspor M. Cold-induced recruitment of brown adipose tissue thermogenesis. Experimental Physiology. 2003;88(1):141–148. - PubMed
-
- Cousin B., Cinti S., Morroni M., Raimbault S., Ricquier D., Pénicaud L. Occurrence of brown adipocytes in rat white adipose tissue : molecular and morphological characterization. Journal of Cell Science. 1992;103:931–942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

