Circuit Models of Low-Dimensional Shared Variability in Cortical Networks
- PMID: 30581012
- PMCID: PMC8238668
- DOI: 10.1016/j.neuron.2018.11.034
Circuit Models of Low-Dimensional Shared Variability in Cortical Networks
Abstract
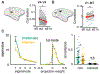
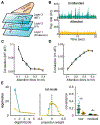
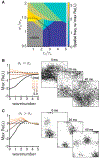
Trial-to-trial variability is a reflection of the circuitry and cellular physiology that make up a neuronal network. A pervasive yet puzzling feature of cortical circuits is that despite their complex wiring, population-wide shared spiking variability is low dimensional. Previous model cortical networks cannot explain this global variability, and rather assume it is from external sources. We show that if the spatial and temporal scales of inhibitory coupling match known physiology, networks of model spiking neurons internally generate low-dimensional shared variability that captures population activity recorded in vivo. Shifting spatial attention into the receptive field of visual neurons has been shown to differentially modulate shared variability within and between brain areas. A top-down modulation of inhibitory neurons in our network provides a parsimonious mechanism for this attentional modulation. Our work provides a critical link between observed cortical circuit structure and realistic shared neuronal variability and its modulation.
Keywords: attention; cortical model; excitatory/inhibitory balance; low dimensional; neuronal variability; noise correlations.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures
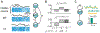



References
-
- Amit DJ, and Brunel N (1997). Model of global spontaneous activity and local structured activity during delay periods in the cerebral cortex. Cereb. Cortex 7, 237–252. - PubMed
-
- Angulo MC, Rossier J, and Audinat E (1999). Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J. Neurophysiol 82, 1295–1302. - PubMed
-
- Börgers C, and Kopell N (2005). Effects of noisy drive on rhythms in networks of excitatory and inhibitory neurons. Neural Comput 17, 557–608. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

