Role of Mitochondria in Ferroptosis
- PMID: 30581146
- PMCID: PMC6338496
- DOI: 10.1016/j.molcel.2018.10.042
Role of Mitochondria in Ferroptosis
Abstract
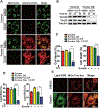
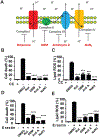
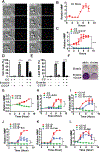
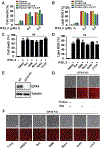
Ferroptosis is a regulated necrosis process driven by iron-dependent lipid peroxidation. Although ferroptosis and cellular metabolism interplay with one another, whether mitochondria are involved in ferroptosis is under debate. Here, we demonstrate that mitochondria play a crucial role in cysteine-deprivation-induced ferroptosis but not in that induced by inhibiting glutathione peroxidase-4 (GPX4), the most downstream component of the ferroptosis pathway. Mechanistically, cysteine deprivation leads to mitochondrial membrane potential hyperpolarization and lipid peroxide accumulation. Inhibition of mitochondrial TCA cycle or electron transfer chain (ETC) mitigated mitochondrial membrane potential hyperpolarization, lipid peroxide accumulation, and ferroptosis. Blockage of glutaminolysis had the same inhibitory effect, which was counteracted by supplying downstream TCA cycle intermediates. Importantly, loss of function of fumarate hydratase, a tumor suppressor and TCA cycle component, confers resistance to cysteine-deprivation-induced ferroptosis. Collectively, this work demonstrates the crucial role of mitochondria in cysteine-deprivation-induced ferroptosis and implicates ferroptosis in tumor suppression.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests (CBT is a founder of Agio Pharmaceuticals and a member of its scientific advisory board. Agio does not hold financial interests in the work reported in this paper).
Figures


References
-
- Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, et al. (2011). Renal Cyst Formation in Fh1-Deficient Mice Is Independent of the Hif/Phd Pathway: Roles for Fumarate in KEAP1 Succination and Nrf2 Signaling. Cancer cell 20, 524–537. - PMC - PubMed
-
- Angeli JPF, Shah R, Pratt DA, and Conrad M (2017). Ferroptosis Inhibition: Mechanisms and Opportunities. Trends in pharmacological sciences 38, 489–498. - PubMed
-
- Cassago A, Ferreira AP, Ferreira IM, Fornezari C, Gomes ER, Greene KS, Pereira HM, Garratt RC, Dias SM, and Ambrosio AL (2012). Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proceedings of the National Academy of Sciences of the United States of America 109, 1092–1097. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

