CSE-1034 versus Ceftriaxone: Efficacy and Safety Analysis from a Randomized, Open-labeled Phase III Study in Complicated Urinary Tract Infections
- PMID: 30581259
- PMCID: PMC6276312
- DOI: 10.4103/jgid.jgid_98_17
CSE-1034 versus Ceftriaxone: Efficacy and Safety Analysis from a Randomized, Open-labeled Phase III Study in Complicated Urinary Tract Infections
Abstract
Objective: The aim of this study was to determine the clinical outcome, microbiological outcome and safety profile of CSE-1034, a novel combination of Ceftriaxone, Sulbactam and EDTA in patients with complicated urinary tract infections (cUTI).
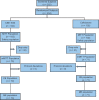
Materials and methods: This was a randomized, controlled, open-labeled Phase-3 trial with the primary objective of assessing the efficacy and safety of CSE-1034 versus Ceftriaxone for the empirical treatment of cUTI. Adult cUTI patients were randomized to receive either intravenous dose of CSE-1034 or Ceftriaxone. The primary end point was composite cure rate (clinical response and bacterial eradication) in mMITT population at test of cure (TOC) visit. Secondary measures included verification of primary endpoint across other visits in different population sets, safety of patients and treatment duration.
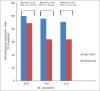
Results: Overall, 204 patients were enrolled in the study and received one of the two treatments. At primary endpoint (TOC visit), the composite cure rate was much higher in CSE-1034 treatment arm compared to Ceftriaxone arm i.e. 97% (68/70) vs 83% (58/71) (treatment difference 12.6%; 95% CI: 5.9% to 26.4%). The adverse events (AEs) rates reported in two treatment arms were 21% in CSE-1034 and 36% in Ceftriaxone groups. Additionally, the treatment duration in CSE-1034 arm was significantly less (P < 0.05).
Conclusions: CSE-1034 3 g every 24 h showed a high favorable clinical and bacteriological response, and 95% CI around the treatment difference prove the superiority of CSE-1034 vs. Ceftriaxone for the treatment of cUTI. Therefore, CSE-1034 provides an effective alternative in the treatment of patients with cUTI.
Keywords: CSE-1034; Ceftriaxone; anti-microbial resistance; cUTI.
Conflict of interest statement
Manu Chaudhary, Shiekh Gazalla Ayub and Mohd Amin Mir are the employees of Venus Remedies, Panchkula. All other authors declare that they have no conflict of interest.
Figures


References
-
- Levison ME, Kaye D. Treatment of complicated urinary tract infections with an emphasis on drug-resistant gram-negative uropathogens. Curr Infect Dis Rep. 2013;15:109–15. - PubMed
-
- Iacovelli V, Gaziev G, Topazio L, Bove P, Vespasiani G, Finazzi Agrò E. Nosocomial urinary tract infections: A review. Urologia. 2014;81:222–27. - PubMed
-
- Pallett A, Hand K. Complicated urinary tract infections: Practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother. 2010;65:iii25–iii33. - PubMed
-
- Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e20. - PubMed

