Action of Colloidal Bismuth Hydroxide Gel and its Commercial Cream on Enteropathogens and Colonizers of the Gastrointestinal Tract
- PMID: 30581262
- PMCID: PMC6276317
- DOI: 10.4103/jgid.jgid_102_17
Action of Colloidal Bismuth Hydroxide Gel and its Commercial Cream on Enteropathogens and Colonizers of the Gastrointestinal Tract
Abstract
Background: Acute diarrheal diseases constitute a world public health problem because they are the second cause of death in children under 5 years of age. Colloidal bismuth hydroxide gel (CBHG) is an active ingredient in low-cost, antidiarrhetic drugs for oral use; it does not inhibit intestinal motility, and it features very low intestinal absorption of <1%.
Materials and methods: We analyzed the sensitivity by determining the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC); the effect on bacterial growth by studying the specific growth velocity and the generation time in growth curves; and bacterial attachment by counting viable plaques, of enteropathogenic Escherichia coli, shigatoxigenic E. coli O157:H7, Klebsiella pneumoniae, Salmonella spp., and Shigella flexneri in the commercial cream (Chobet® bismuth cream with pectin [CBCHP]), its active ingredient (CBHG), and its excipients (E) separately.
Results: CBCHP: MIC 6-10 mg/ml and MBC 7.5-15 mg/ml of bismuth; CBHG: MIC 6-10 mg/ml of bismuth. E: No inhibition was observed at the concentration studied in this study. At very low subinhibitory concentrations of CBCHP and CBHG, there was already evidence of a significant decrease in growth, which could not be recorded for E. CBCHP and CBHG presented an elevated capacity for bacterial displacement, significantly greater than E.
Conclusions: We believed that the results obtained in this study are very promising from the treatment standpoint, as a possible treatment for cases of diagnosis or suspicion of bacterial gastroenteritis. The antimicrobial and attachment effects of CBCHP are exclusively due to its active ingredient CBHG; these effects are promoted in the presence of E.
Keywords: Colloidal bismuth; diarrhea; enteropathogens.
Conflict of interest statement
There are no conflicts of interest.
Figures
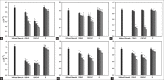
 ); 0.06 mg/ml (
); 0.06 mg/ml ( ); 0.12 mg/ml (
); 0.12 mg/ml ( ); 0.3 mg/ml (
); 0.3 mg/ml ( ) and 0.6 mg/ml (
) and 0.6 mg/ml ( ). *(P < 0.05), **(P < 0.01)
). *(P < 0.05), **(P < 0.01)
 ); 0.06 mg/ml (
); 0.06 mg/ml ( ); 0.12 mg/ml (
); 0.12 mg/ml ( ); 0.3 mg/ml (
); 0.3 mg/ml ( ) and 0.6 mg/ml (
) and 0.6 mg/ml ( ). *(P < 0.05), **(P < 0.01)
). *(P < 0.05), **(P < 0.01)Similar articles
-
The inhibitory effect of colloidal bismuth hydroxide gel on Escherichia coli O157:H7 and on the activity of Shiga toxins.BMC Res Notes. 2014 Dec 4;7:875. doi: 10.1186/1756-0500-7-875. BMC Res Notes. 2014. PMID: 25475210 Free PMC article.
-
Acute Diarrhoea in Children: Determination of Duration Using a Combined Bismuth Hydroxide Gel and Oral Rehydration Solution Therapy vs. Oral Rehydration Solution.Children (Basel). 2016 Dec 21;3(4):45. doi: 10.3390/children3040045. Children (Basel). 2016. PMID: 28009823 Free PMC article.
-
Prevalence of Escherichia coli O157:H7 in Children with Bloody Diarrhea Referring to Abuzar Teaching Hospital, Ahvaz, Iran.J Clin Diagn Res. 2016 Jan;10(1):DC13-5. doi: 10.7860/JCDR/2016/16689.7134. Epub 2016 Jan 1. J Clin Diagn Res. 2016. PMID: 26894066 Free PMC article.
-
Nonfluid therapy and selected chemoprophylaxis of acute diarrhea.Am J Med. 1985 Jun 28;78(6B):81-90. doi: 10.1016/0002-9343(85)90369-9. Am J Med. 1985. PMID: 3893119 Review.
-
Bismuth subsalicylate in the treatment and prevention of diarrheal disease.Drug Intell Clin Pharm. 1987 Sep;21(9):687-93. doi: 10.1177/106002808702100901. Drug Intell Clin Pharm. 1987. PMID: 3308391 Review.
References
-
- World Health Organization. World Health Statistics 2011. World Health Organization. 2011. Available from: http://www.who.int/gho/publications/world_health_statistics/EN_WHS2011_F... .
-
- Giugno S, Oderiz S. Bacterial etiology of acute diarrhea in pediatric patients. Acta Bioquím Clín Latinoam. 2010;44:63–9.
-
- Pan American Health Organization. Health in the Americas. Argentina: Pan American Health Organization; 2012. [Last accessed on 2015 May 14]. Available from: http://www.paho.org/salud.en.las.americas.2012/index.php?option=com_cont... .
-
- González Corona E, Cunil Romero S. Diagnosis and treatment of persistent diarrhea in the service of acute diarrheic diseases. Medisan. 2002;6:42–9.
-
- Casabonne C, González A, Aquili V, Balagué C. Prevalence and virulence genes of Shigella spp. Isolated from patients with diarrhea in Rosario, Argentina. Jpn J Infect Dis. 2016;69:477–81. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

