Relationship between response to lusutrombopag and splenic volume
- PMID: 30581275
- PMCID: PMC6295839
- DOI: 10.3748/wjg.v24.i46.5271
Relationship between response to lusutrombopag and splenic volume
Abstract
Aim: To assess the correlation between the efficacy of lusutrombopag and clinical characteristics in patients with chronic liver disease.
Methods: In this retrospective, multicenter study, which conducted at four locations in Japan, 50 thrombocytopenic patients with chronic liver disease were enrolled. All patients received oral lusutrombopag (3.0 mg/d for 7 d) for chronic liver disease. We assessed the increase in platelet count after the trial drug administration. A treatment response was defined as a platelet count ≥ 5 × 104/μL and an increased platelet count ≥ 2 × 104/μL from baseline after drug administration. We evaluated the response to lusutrombopag compared to baseline clinical characteristics in patients with chronic liver disease.
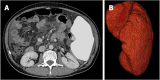
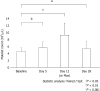
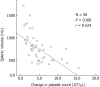
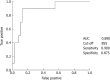
Results: The numbers of responders and non-responders were 40 (80.0%) and 10 (20.0%), respectively. The patients were divided into a responder and non-responder group, and we added factors that may correspond to successful treatment with lusutrombopag. Splenic volume and body weight were lower in the responder group than in the non-responder group. White blood cell count and hemoglobin level were higher in responders compared with non-responders. Using a logistic regression model to assess the relationship between response to lusutrombopag and clinical characteristics, multivariate analysis confirmed that splenic volume was an independent factor that predicted the response of platelet counts (P = 0.025; odds ratio = 11.2; 95% confidence interval: 1.354-103.0). Splenic volume negatively correlated to changes in platelet count (r = -0.524, P = 0.001).
Conclusion: Splenic volume influences the change in platelet counts after administration of lusutrombopag in patients with chronic liver disease.
Keywords: Lusutrombopag; Portal hypertension; Splenic volume; Thrombocytopenia; Thrombopoietin receptor agonist.
Conflict of interest statement
Conflict-of-interest statement: We declare no conflicts of interest.
Figures
Similar articles
-
Avatrombopag and lusutrombopag for thrombocytopenia in people with chronic liver disease needing an elective procedure: a systematic review and cost-effectiveness analysis.Health Technol Assess. 2020 Oct;24(51):1-220. doi: 10.3310/hta24510. Health Technol Assess. 2020. PMID: 33108266 Free PMC article.
-
The Thrombopoietin Receptor Agonist Lusutrombopag Is Effective for Patients with Chronic Liver Disease and Impaired Renal Function.J Nippon Med Sch. 2021 Jan 8;87(6):325-333. doi: 10.1272/jnms.JNMS.2020_87-603. Epub 2020 Mar 31. J Nippon Med Sch. 2021. PMID: 32238734
-
Impact of Anti-GPIIb/IIIa Antibody-Producing B Cells as a Predictor of the Response to Lusutrombopag in Thrombocytopenic Patients with Liver Disease.Dig Dis. 2021;39(3):234-242. doi: 10.1159/000510692. Epub 2020 Aug 6. Dig Dis. 2021. PMID: 32759604 Free PMC article. Clinical Trial.
-
Effectiveness of Lusutrombopag in Patients with Mild to Moderate Thrombocytopenia.Dig Dis. 2020;38(4):329-334. doi: 10.1159/000504044. Epub 2019 Oct 25. Dig Dis. 2020. PMID: 31655803 Clinical Trial.
-
Lusutrombopag: A Review in Thrombocytopenia in Patients with Chronic Liver Disease Prior to a Scheduled Procedure.Drugs. 2019 Oct;79(15):1689-1695. doi: 10.1007/s40265-019-01197-8. Drugs. 2019. PMID: 31529283 Review.
Cited by
-
Efficacy of Lusutrombopag for Thrombocytopenia in Patients with Chronic Liver Disease Scheduled to Undergo Invasive Procedures.Intern Med. 2021 Mar 15;60(6):829-837. doi: 10.2169/internalmedicine.5930-20. Epub 2020 Oct 21. Intern Med. 2021. PMID: 33087674 Free PMC article.
-
Thrombocytopenia in chronic liver disease: Physiopathology and new therapeutic strategies before invasive procedures.World J Gastroenterol. 2022 Aug 14;28(30):4061-4074. doi: 10.3748/wjg.v28.i30.4061. World J Gastroenterol. 2022. PMID: 36157107 Free PMC article. Review.
-
Assessing the periprocedural magnitude of platelet count change in response to lusutrombopag.JHEP Rep. 2021 Jan 13;3(2):100228. doi: 10.1016/j.jhepr.2021.100228. eCollection 2021 Apr. JHEP Rep. 2021. PMID: 33644726 Free PMC article.
-
Safety and effectiveness of lusutrombopag in Japanese chronic liver disease patients with thrombocytopenia undergoing invasive procedures: Interim results of a postmarketing surveillance.Hepatol Res. 2019 Oct;49(10):1169-1181. doi: 10.1111/hepr.13392. Epub 2019 Jul 22. Hepatol Res. 2019. PMID: 31228221 Free PMC article.
-
Evaluation of the Efficacy of Lusutrombopag for Chronic Liver Disease Based on Pre-Treatment Platelet Counts: A Retrospective Multicenter Study.JGH Open. 2024 Dec 31;9(1):e70081. doi: 10.1002/jgh3.70081. eCollection 2025 Jan. JGH Open. 2024. PMID: 39742152 Free PMC article.
References
-
- Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–1007. - PubMed
-
- Poordad F. Review article: thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther. 2007;26 Suppl 1:5–11. - PubMed
-
- Stiegler G, Stohlawetz P, Peck-Radosavljevic M, Jilma B, Pidlich J, Wichlas M, Höcker P, Panzer S. Direct evidence for an increase in thrombopoiesis after liver transplantation. Eur J Clin Invest. 1998;28:755–759. - PubMed
-
- Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14 Suppl D:60D–66D. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

