Preliminary application of 3D-printed coplanar template for iodine-125 seed implantation therapy in patients with advanced pancreatic cancer
- PMID: 30581276
- PMCID: PMC6295836
- DOI: 10.3748/wjg.v24.i46.5280
Preliminary application of 3D-printed coplanar template for iodine-125 seed implantation therapy in patients with advanced pancreatic cancer
Abstract
Aim: To evaluate a 3D-printed coplanar template for iodine-125 seed implantation therapy in patients with pancreatic cancer.
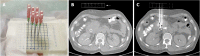
Methods: A retrospective analysis of our database was performed, and a total of 25 patients with pancreatic cancer who underwent iodine-125 seed implantation between January 2014 and November 2017 were analyzed. Of these, 12 implantations were assisted by a 3D-printed coplanar template (group A), and 13 implantations performed freehand were selected as a control group (group B). A 3D coplanar template was designed and printed according to a preoperative CT scan and treatment planning system. The iodine-125 seeds were then implanted using the template as a guide. Dosimetric verification was performed after implantation. Pre- and postoperative D90, V100, and V150 were calculated. The success rate of iodine-125 seed implantation, dosimetric parameters, and complications were analyzed and compared between the two groups.
Results: Iodine-125 seed implantation was successfully performed in both groups. In group A, the median pre- and postoperative D90 values were 155.32 ± 8.05 Gy and 154.82 ± 16.43 Gy, respectively; the difference between these values was minimal and not statistically significant (P > 0.05). Postoperative V100 and V150 were 91.05% ± 4.06% and 64.54% ± 13.40%, respectively, which met the treatment requirement. A better dosimetric parameter was observed in group A than in group B, and the difference was statistically significant (V100: 91.05% ± 4.06% vs 72.91% ± 13.78%, P < 0.05). No major procedure-related complications were observed in either group. For group A, mild hemorrhage was observed in 1 patient with a peritoneal local hematoma due to mesenteric vein damage from the iodine-125 seed implantation needle. The hematoma resolved spontaneously without treatment. Postoperative blood amylase levels remained within the normal range for all patients.
Conclusion: A 3D-printed coplanar template appears to be a safe and effective iodine-125 seed implantation guidance tool to improve implantation accuracy and optimize dosimetric distribution.
Keywords: 3D printing; Brachytherapy; Iodine-125; Pancreatic cancer.
Conflict of interest statement
Conflict-of-interest statement: None declared.
Figures


References
-
- Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. - PubMed
-
- Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–5518. - PubMed
-
- Colucci G, Labianca R, Di Costanzo F, Gebbia V, Cartenì G, Massidda B, Dapretto E, Manzione L, Piazza E, Sannicolò M, Ciaparrone M, Cavanna L, Giuliani F, Maiello E, Testa A, Pederzoli P, Falconi M, Gallo C, Di Maio M, Perrone F; Gruppo Oncologico Italia Meridionale (GOIM); Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD); Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC) Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645–1651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

