A Newly Authenticated Compound from Traditional Chinese Medicine Decoction Induces Melanogenesis in B16-F10 Cells by Increasing Tyrosinase Activity
- PMID: 30581488
- PMCID: PMC6276395
- DOI: 10.1155/2018/8485670
A Newly Authenticated Compound from Traditional Chinese Medicine Decoction Induces Melanogenesis in B16-F10 Cells by Increasing Tyrosinase Activity
Abstract
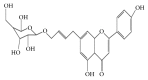
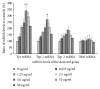
Vitiligo is a kind of skin dysfunction on melanogenesis. The highly prevalent, chronic, and distinctive complexion changes on patients have imposed enormous psychic and economic burden on both individuals and society. Traditional Chinese Medicine (TCM) is a kind of precious source on chronic disease treatment, including skin dysfunctional diseases. In our previous study, a new compound named apigenin-7-butylene glucoside has been authenticated and purified from a prescription of Chinese traditional medicine formula which has been used clinically in vitiligo treatment. The aim of this work is to evaluate the effects of this compound on melanogenesis using melanoma cell B16-F10 in vitro. The results showed that apigenin-7-butylene glucoside had almost no cytotoxicity on B16-F10 cells within a lower dose of 5.0 μg ml-1 and enhanced the melanin level to about 41% and tyrosinase activity to 1.32-fold when compared with controls. The compound showed minor cytotoxicity to B16-F10 cells at the higher concentration of 10 μg ml-1 and 50 μg ml-1, the inhibition rate was 8.4% and 11.8%, and the melanin level and tyrosinase activity showed a decreased trend because of the lower cell number at the higher concentrations. The results indicated that apigenin-7-butylene glucoside was safe to B16-F10 cells within a lower concentration, <5.0 μg ml-1. Incubated with 5.0 ug ml-1of apigenin-7-butylene glucoside for 48 hours, the mRNA and protein levels of Tyr, Trp-1, and Trp-2 genes were all increased except Mitf in B16-F10 cells. The stimulation of apigenin-7-butylene glucoside on melanogenesis of B16-F10 cells through Tyr, Trp-1, and Trp-2 pathway highlighted the potential usage of the compound in vitiligo treatment.
Figures




Similar articles
-
Inhibition of melanogenesis in murine B16/F10 melanoma cells by Ligusticum sinensis Oliv.Am J Chin Med. 2006;34(3):523-33. doi: 10.1142/S0192415X06004053. Am J Chin Med. 2006. PMID: 16710901
-
Activation of neurokinin-1 receptor by substance P inhibits melanogenesis in B16-F10 melanoma cells.Int J Biochem Cell Biol. 2012 Dec;44(12):2342-8. doi: 10.1016/j.biocel.2012.09.025. Epub 2012 Oct 2. Int J Biochem Cell Biol. 2012. PMID: 23041339
-
Identification of Anti-Melanogenesis Constituents from Morus alba L. Leaves.Molecules. 2018 Oct 8;23(10):2559. doi: 10.3390/molecules23102559. Molecules. 2018. PMID: 30297610 Free PMC article.
-
Diethylstilbestrol enhances melanogenesis via cAMP-PKA-mediating up-regulation of tyrosinase and MITF in mouse B16 melanoma cells.Steroids. 2011 Nov;76(12):1297-304. doi: 10.1016/j.steroids.2011.06.008. Epub 2011 Jun 30. Steroids. 2011. PMID: 21745488
-
Peroxisome proliferator-activated receptor α (PPARα) contributes to control of melanogenesis in B16 F10 melanoma cells.Arch Dermatol Res. 2017 Apr;309(3):141-157. doi: 10.1007/s00403-016-1711-2. Epub 2017 Jan 13. Arch Dermatol Res. 2017. PMID: 28084540
Cited by
-
Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials.Antioxidants (Basel). 2020 Jul 18;9(7):637. doi: 10.3390/antiox9070637. Antioxidants (Basel). 2020. PMID: 32708455 Free PMC article. Review.
-
Characterization and Functional Analysis of a Novel Fungal Immunomodulatory Protein Gene from Ganoderma leucocontextum in B16-F10 Mouse Melanoma Cells.Int J Mol Sci. 2025 May 24;26(11):5063. doi: 10.3390/ijms26115063. Int J Mol Sci. 2025. PMID: 40507874 Free PMC article.
-
Skin Improvement Effects of Ultrasound-Enzyme-Treated Collagen Peptide Extracts from Flatfish (Paralichthys olivaceus) Skin in an In Vitro Model.Int J Mol Sci. 2024 Aug 27;25(17):9300. doi: 10.3390/ijms25179300. Int J Mol Sci. 2024. PMID: 39273248 Free PMC article.
-
Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources.Molecules. 2020 Mar 27;25(7):1537. doi: 10.3390/molecules25071537. Molecules. 2020. PMID: 32230973 Free PMC article. Review.
-
Tricholoma matsutake polysaccharides suppress excessive melanogenesis via JNK-mediated pathway: Investigation in 8- methoxypsoralen induced B16-F10 melanoma cells and clinical study.Heliyon. 2024 Apr 8;10(8):e29363. doi: 10.1016/j.heliyon.2024.e29363. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38644864 Free PMC article.
References
-
- Sahni K., Parsad D., Kanwar A. J., Mehta S. D. Autologous noncultured melanocyte transplantation for stable vitiligo: Can suspending autologous melanocytes in the patients' own serum improve repigmentation and patient satisfaction? Dermatologic Surgery. 2011;37(2):176–182. doi: 10.1111/j.1524-4725.2010.01847.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

