Sulforaphane Modulates AQP8-Linked Redox Signalling in Leukemia Cells
- PMID: 30581529
- PMCID: PMC6276444
- DOI: 10.1155/2018/4125297
Sulforaphane Modulates AQP8-Linked Redox Signalling in Leukemia Cells
Abstract
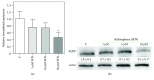
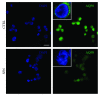
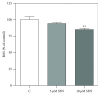
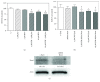
Sulforaphane, a biologically active isothiocyanate compound extracted from cruciferous vegetables, has been shown to exert cytotoxic effects on many human cancer cells, including leukemia. However, the exact molecular mechanisms behind the action of sulforaphane in hematological malignancies are still unclear. Like other cancer cells, leukemia cells produce high level of reactive oxygen species; in particular, hydrogen peroxide derived from Nox family is involved in various redox signal transduction pathways, promoting cell proliferation and survival. Recent evidence show that many tumour cell types express elevated level of aquaporin isoforms, and we previously demonstrated that aquaporin-8 acts as H2O2 transport facilitator across the plasma membrane of B1647 cells, a model of acute myeloid human leukemia. Thus, the control of AQP8-mediated H2O2 transport could be a novel strategy to regulate cell signalling and survival. To this purpose, we evaluated whether sulforaphane could somehow affect aquaporin-8-mediated H2O2 transport and/or Nox-mediated H2O2 production in B1647 cell line. Results indicated that sulforaphane inhibited both aquaporin-8 and Nox2 expression, thus decreasing B1647 cells viability. Moreover, the data obtained by coimmunoprecipitation technique demonstrated that these two proteins are linked to each other; thus, sulforaphane has an important role in modulating the downstream events triggered by the axis Nox2-aquaporin-8. Cell treatment with sulforaphane also reduced the expression of peroxiredoxin-1, which is increased in almost all acute myeloid leukemia subtypes. Interestingly, sulforaphane concentrations able to trigger these effects are achievable by dietary intake of cruciferous vegetables, confirming the importance of the beneficial effect of a diet rich in bioactive compounds.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

