Enhanced Raman Investigation of Cell Membrane and Intracellular Compounds by 3D Plasmonic Nanoelectrode Arrays
- PMID: 30581692
- PMCID: PMC6299714
- DOI: 10.1002/advs.201800560
Enhanced Raman Investigation of Cell Membrane and Intracellular Compounds by 3D Plasmonic Nanoelectrode Arrays
Abstract
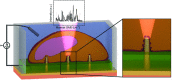
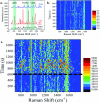
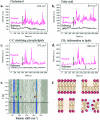
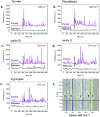
3D nanostructures are widely exploited in cell cultures for many purposes such as controlled drug delivery, transfection, intracellular sampling, and electrical recording. However, little is known about the interaction of the cells with these substrates, and even less about the effects of electroporation on the cellular membrane and the nuclear envelope. This work exploits 3D plasmonic nanoelectrodes to study, by surface-enhanced Raman scattering (SERS), the cell membrane dynamics on the nanostructured substrate before, during, and after electroporation. In vitro cultured cells tightly adhere on 3D plasmonic nanoelectrodes precisely in the plasmonic hot spots, making this kind of investigation possible. After electroporation, the cell membrane dynamics are studied by recording the Raman time traces of biomolecules in contact or next to the 3D plasmonic nanoelectrode. During this process, the 3D plasmonic nanoelectrodes are intracellularly coupled, thus enabling the monitoring of different molecular species, including lipids, proteins, and nucleic acids. Scanning electron microscopy cross-section analysis evidences the possibility of nuclear membrane poration compatible with the reported Raman spectra. These findings may open a new route toward controlled intracellular sampling and intranuclear delivery of genic materials. They also show the possibility of nuclear envelope disruption which may lead to negative side effects.
Keywords: Raman spectroscopy; electroporation; intracellular spectroscopy; microelectrode arrays; nanopillars; permeabilization.
Figures

Similar articles
-
On-Demand Intracellular Delivery of Single Particles in Single Cells by 3D Hollow Nanoelectrodes.Nano Lett. 2019 Feb 13;19(2):722-731. doi: 10.1021/acs.nanolett.8b03764. Epub 2019 Jan 23. Nano Lett. 2019. PMID: 30673248 Free PMC article.
-
Intracellular and Extracellular Recording of Spontaneous Action Potentials in Mammalian Neurons and Cardiac Cells with 3D Plasmonic Nanoelectrodes.Nano Lett. 2017 Jun 14;17(6):3932-3939. doi: 10.1021/acs.nanolett.7b01523. Epub 2017 May 24. Nano Lett. 2017. PMID: 28534411 Free PMC article.
-
Out-of-Plane Plasmonic Antennas for Raman Analysis in Living Cells.Small. 2015 Sep;11(36):4632-7. doi: 10.1002/smll.201500891. Epub 2015 Jun 26. Small. 2015. PMID: 26114644
-
Plasmonic substrates for biochemical applications of surface-enhanced Raman spectroscopy.Spectrochim Acta A Mol Biomol Spectrosc. 2024 Mar 5;308:123786. doi: 10.1016/j.saa.2023.123786. Epub 2023 Dec 16. Spectrochim Acta A Mol Biomol Spectrosc. 2024. PMID: 38128327 Review.
-
Substrates for Surface-Enhanced Raman Scattering Formed on Nanostructured Non-Metallic Materials: Preparation and Characterization.Nanomaterials (Basel). 2020 Dec 31;11(1):75. doi: 10.3390/nano11010075. Nanomaterials (Basel). 2020. PMID: 33396325 Free PMC article. Review.
Cited by
-
Resonant Enhancement of Polymer-Cell Optostimulation by a Plasmonic Metasurface.ACS Omega. 2022 Nov 16;7(47):42674-42680. doi: 10.1021/acsomega.2c04812. eCollection 2022 Nov 29. ACS Omega. 2022. PMID: 36467911 Free PMC article. Review.
-
High-Aspect-Ratio Nanostructured Surfaces as Biological Metamaterials.Adv Mater. 2020 Mar;32(9):e1903862. doi: 10.1002/adma.201903862. Epub 2020 Jan 16. Adv Mater. 2020. PMID: 31944430 Free PMC article. Review.
-
Plasmonic Metasurface for Spatially Resolved Optical Sensing in Three Dimensions.ACS Nano. 2020 Feb 25;14(2):2345-2353. doi: 10.1021/acsnano.9b09508. Epub 2020 Feb 3. ACS Nano. 2020. PMID: 31986008 Free PMC article.
-
Cellular nanointerface of vertical nanostructure arrays and its applications.Nanoscale Adv. 2022 Feb 21;4(8):1844-1867. doi: 10.1039/d1na00775k. eCollection 2022 Apr 12. Nanoscale Adv. 2022. PMID: 36133409 Free PMC article. Review.
-
Present and Future of Surface-Enhanced Raman Scattering.ACS Nano. 2020 Jan 28;14(1):28-117. doi: 10.1021/acsnano.9b04224. Epub 2019 Oct 8. ACS Nano. 2020. PMID: 31478375 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
