Gene Transfer with AAV9-PHP.B Rescues Hearing in a Mouse Model of Usher Syndrome 3A and Transduces Hair Cells in a Non-human Primate
- PMID: 30581889
- PMCID: PMC6297893
- DOI: 10.1016/j.omtm.2018.11.003
Gene Transfer with AAV9-PHP.B Rescues Hearing in a Mouse Model of Usher Syndrome 3A and Transduces Hair Cells in a Non-human Primate
Abstract
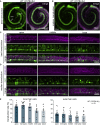
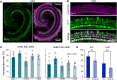
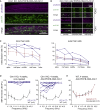
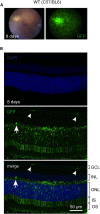
Hereditary hearing loss often results from mutation of genes expressed by cochlear hair cells. Gene addition using AAV vectors has shown some efficacy in mouse models, but clinical application requires two additional advances. First, new AAV capsids must mediate efficient transgene expression in both inner and outer hair cells of the cochlea. Second, to have the best chance of clinical translation, these new vectors must also transduce hair cells in non-human primates. Here, we show that an AAV9 capsid variant, PHP.B, produces efficient transgene expression of a GFP reporter in both inner and outer hair cells of neonatal mice. We show also that AAV9-PHP.B mediates almost complete transduction of inner and outer HCs in a non-human primate. In a mouse model of Usher syndrome type 3A deafness (gene CLRN1), we use AAV9-PHP.B encoding Clrn1 to partially rescue hearing. Thus, we have identified a vector with promise for clinical treatment of hereditary hearing disorders, and we demonstrate, for the first time, viral transduction of the inner ear of a primate with an AAV vector.
Keywords: AAV; adeno-associated virus vector; cochlea; gene delivery; hair cells; hereditary deafness; inner ear; non-human primate.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

