Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial
- PMID: 30582156
- PMCID: PMC7379690
- DOI: 10.1111/epi.14628
Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial
Abstract
Objective: Add-on cannabidiol (CBD) significantly reduced seizures associated with Dravet syndrome (DS) in a randomized, double-blind, placebo-controlled trial: GWPCARE1 Part B (NCT02091375). Patients who completed GWPCARE1 Part A (NCT02091206) or Part B, or a second placebo-controlled trial, GWPCARE2 (NCT02224703), were invited to enroll in a long-term open-label extension trial, GWPCARE5 (NCT02224573). We present an interim analysis of the safety, efficacy, and patient-reported outcomes from GWPCARE5.
Methods: Patients received a pharmaceutical formulation of highly purified CBD in oral solution (100 mg/mL), titrated from 2.5 to 20 mg/kg/d over a 2-week period, with their existing medications. Based on response and tolerance, CBD could be reduced or increased up to 30 mg/kg/d.
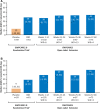
Results: By November 2016, a total of 278 patients had completed the original randomized trials, and 264 (95%) enrolled in this open-label extension. Median treatment duration was 274 days (range 1-512) with a mean modal dose of 21 mg/kg/d, and patients received a median of 3 concomitant antiepileptic medications. Adverse events (AEs) occurred in 93.2% of patients and were mostly mild (36.7%) or moderate (39.0%). Commonly reported AEs were diarrhea (34.5%), pyrexia (27.3%), decreased appetite (25.4%), and somnolence (24.6%). Seventeen patients (6.4%) discontinued due to AEs. Twenty-two of the 128 patients from GWPCARE1 (17.2%), all taking valproic acid, had liver transaminase elevations ≥3 times the upper limit of normal. In patients from GWPCARE1 Part B, the median reduction from baseline in monthly seizure frequency assessed in 12-week periods up to week 48 ranged from 38% to 44% for convulsive seizures and 39% to 51% for total seizures. After 48 weeks of treatment, 85% of patients/caregivers reported improvement in the patient's overall condition on the Subject/Caregiver Global Impression of Change scale.
Significance: This trial shows that long-term CBD treatment had an acceptable safety profile and led to sustained, clinically meaningful reductions in seizure frequency in patients with treatment-resistant DS.
Keywords: cannabinoid; epileptic encephalopathy; seizures; treatment-resistant epilepsy.
© 2018 The Authors. Epilepsia published by Wiley Periodicals, Inc. on behalf of International League Against Epilepsy.
Conflict of interest statement
Orrin Devinsky has served as a consultant/advisor to GW Pharmaceuticals and Pairnomix, and as a study investigator for GW Pharmaceuticals, and has equity interest in Tevard, Empatica, Privateer Holdings, and Receptor Life Sciences. Rima Nabbout has served as a consultant/advisor/lecturer for Novartis, Zogenix, Nutricia, Advicennes, Eisai, GW Pharmaceuticals and as a study investigator for GW Pharmaceuticals, Advicennes, UCB, Eisai, and Zogenix. Ian Miller has served as a consultant/advisor to GW Pharmaceuticals, Insys Therapeutics, Visualase, and NeuroPace, and as a study investigator for GW Pharmaceuticals. Linda Laux is a study investigator for GW Pharmaceuticals and Zogenix. Marta Zolnowska is a study investigator for GW Pharmaceuticals. Stephen Wright is employed by GW Research Ltd. Claire Roberts was employed by GW Research Ltd at the time of this study, and is now affiliated with Eisai Ltd. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures



References
-
- Dravet C, Bureau M, Oguni H, et al. Dravet syndrome (severe myoclonic epilepsy in infancy) In: Bureau M, Genton P, Dravet C, et al. editors. Epileptic syndromes in infancy, childhood and adolescence. Montrouge, France: John Libbey Eurotext, 2012; p. 125–56.
-
- Bayat A, Hjalgrim H, Moller RS. The incidence of SCN1A‐related Dravet syndrome in Denmark is 1:22,000: a population‐based study from 2004 to 2009. Epilepsia. 2015;56:e36–9. - PubMed
-
- Brunklaus A, Ellis R, Reavey E, et al. Prognostic, clinical and demographic features in SCN1A mutation‐positive Dravet syndrome. Brain. 2012;135:2329–36. - PubMed
-
- Rosander C, Hallbook T. Dravet syndrome in Sweden: a population‐based study. Dev Med Child Neurol. 2015;57:628–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

