Comparison of the effects of standard vs low-dose prolonged-release tacrolimus with or without ACEi/ARB on the histology and function of renal allografts
- PMID: 30582281
- PMCID: PMC6590452
- DOI: 10.1111/ajt.15225
Comparison of the effects of standard vs low-dose prolonged-release tacrolimus with or without ACEi/ARB on the histology and function of renal allografts
Abstract
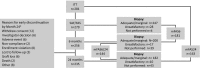
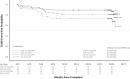
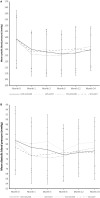
Targeting the renin-angiotensin system and optimizing tacrolimus exposure are both postulated to improve outcomes in renal transplant recipients (RTRs) by preventing interstitial fibrosis/tubular atrophy (IF/TA). In this multicenter, prospective, open-label controlled trial, adult de novo RTRs were randomized in a 2 × 2 design to low- vs standard-dose (LOW vs STD) prolonged-release tacrolimus and to angiotensin-converting enzyme inhibitors/angiotensin II receptor 1 blockers (ACEi/ARBs) vs other antihypertensive therapy (OAHT). There were 2 coprimary endpoints: the prevalence of IF/TA at month 6 and at month 24. IF/TA prevalence was similar for LOW vs STD tacrolimus at month 6 (36.8% vs 39.5%; P = .80) and ACEi/ARBs vs OAHT at month 24 (54.8% vs 58.2%; P = .33). IF/TA progression decreased significantly with LOW vs STD tacrolimus at month 24 (mean [SD] change, +0.42 [1.477] vs +1.10 [1.577]; P = .0039). Across the 4 treatment groups, LOW + ACEi/ARB patients exhibited the lowest mean IF/TA change and, compared with LOW + OAHT patients, experienced significantly delayed time to first T cell-mediated rejection. Renal function was stable from month 1 to month 24 in all treatment groups. No unexpected safety findings were detected. Coupled with LOW tacrolimus dosing, ACEi/ARBs appear to reduce IF/TA progression and delay rejection relative to reduced tacrolimus exposure without renin-angiotensin system blockade. ClinicalTrials.gov identifier: NCT00933231.
Keywords: clinical research/practice; clinical trial; graft survival; immunosuppressant - calcineurin inhibitor: tacrolimus; immunosuppression/immune modulation; kidney transplantation/nephrology; organ transplantation in general; patient survival.
© 2018 Astellas Pharma, Inc. American Journal of Transplantation published by Wiley Periodicals, Inc. on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures







References
-
- Lamb KE, Lodhi S, Meier‐Kriesche H‐U. Long‐term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11(3):450‐462. - PubMed
-
- Lodhi SA, Meier‐Kriesche H‐U. Kidney allograft survival: the long and short of it. J Lab Clin Med Nephrol Dial Transplant. 2011;26(1):15‐17. - PubMed
-
- Knoll G. Trends in kidney transplantation over the past decade. Drugs. 2008;68(suppl 1):3‐10. - PubMed
-
- Rush D, Arlen D, Boucher A, et al. Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: a randomized study. Am J Transplant. 2007;7(11):2538‐2545. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

