Redox Biology of Peroxisome Proliferator-Activated Receptor-γ in Pulmonary Hypertension
- PMID: 30582337
- PMCID: PMC6751396
- DOI: 10.1089/ars.2018.7695
Redox Biology of Peroxisome Proliferator-Activated Receptor-γ in Pulmonary Hypertension
Abstract
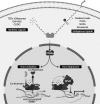
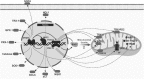
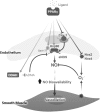
Significance: Peroxisome proliferator-activated receptor-gamma (PPARγ) maintains pulmonary vascular health through coordination of antioxidant defense systems, inflammation, and cellular metabolism. Insufficient PPARγ contributes to pulmonary hypertension (PH) pathogenesis, whereas therapeutic restoration of PPARγ activity attenuates PH in preclinical models. Recent Advances: Numerous studies in the past decade have elucidated the complex mechanisms by which PPARγ in the pulmonary vasculature and right ventricle (RV) protects against PH. The scope of PPARγ-interconnected pathways continues to expand and includes induction of antioxidant genes, transrepression of inflammatory signaling, regulation of mitochondrial biogenesis and bioenergetic integrity, control of cell cycle and proliferation, and regulation of vascular tone through interactions with nitric oxide and endogenous vasoactive molecules. Furthermore, PPARγ interacts with an extensive regulatory network of transcription factors and microRNAs leading to broad impact on cell signaling. Critical Issues: Abundant evidence suggests that targeting PPARγ exerts diverse salutary effects in PH and represents a novel and potentially translatable therapeutic strategy. However, progress has been slowed by an incomplete understanding of how specific PPARγ pathways are critically disrupted across PH disease subtypes and lack of optimal pharmacological ligands. Future Directions: Recent studies indicate that ligand-induced post-translational modifications of the PPARγ receptor differentially induce therapeutic benefits versus adverse side effects of PPARγ receptor activation. Strategies to selectively target PPARγ activity in diseased cells of pulmonary circulation and RV, coupled with development of ligands designed to specifically regulate post-translational PPARγ modifications, may unlock the full therapeutic potential of this versatile master transcriptional and metabolic regulator in PH.
Keywords: PPARγ; antioxidants; hypoxia; oxidative stress; pulmonary hypertension; thiazolidinedione.
Conflict of interest statement
Drs. Tseng, Sutliff, and Hart have no actual or potential conflicts of interest to disclose and no competing financial interests exist.
Figures




Similar articles
-
Peroxisome Proliferator-Activated Receptor γ and microRNA 98 in Hypoxia-Induced Endothelin-1 Signaling.Am J Respir Cell Mol Biol. 2016 Jan;54(1):136-46. doi: 10.1165/rcmb.2014-0337OC. Am J Respir Cell Mol Biol. 2016. PMID: 26098770 Free PMC article.
-
Hypoxia mediates mutual repression between microRNA-27a and PPARγ in the pulmonary vasculature.PLoS One. 2013 Nov 14;8(11):e79503. doi: 10.1371/journal.pone.0079503. eCollection 2013. PLoS One. 2013. PMID: 24244514 Free PMC article.
-
PPARγ Regulates Mitochondrial Structure and Function and Human Pulmonary Artery Smooth Muscle Cell Proliferation.Am J Respir Cell Mol Biol. 2018 May;58(5):648-657. doi: 10.1165/rcmb.2016-0293OC. Am J Respir Cell Mol Biol. 2018. PMID: 29182484 Free PMC article.
-
PPARgamma as a potential therapeutic target in pulmonary hypertension.Ther Adv Respir Dis. 2010 Jun;4(3):143-60. doi: 10.1177/1753465809369619. Ther Adv Respir Dis. 2010. PMID: 20530063 Free PMC article. Review.
-
Evidence that peroxisome proliferator-activated receptor γ suppresses squamous carcinogenesis through anti-inflammatory signaling and regulation of the immune response.Mol Carcinog. 2019 Sep;58(9):1589-1601. doi: 10.1002/mc.23041. Epub 2019 May 20. Mol Carcinog. 2019. PMID: 31111568 Review.
Cited by
-
PPARγ/SOD2 Protects Against Mitochondrial ROS-Dependent Apoptosis via Inhibiting ATG4D-Mediated Mitophagy to Promote Pancreatic Cancer Proliferation.Front Cell Dev Biol. 2022 Feb 2;9:745554. doi: 10.3389/fcell.2021.745554. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35186942 Free PMC article.
-
Deletion of narfl leads to increased oxidative stress mediated abnormal angiogenesis and digestive organ defects in zebrafish.Redox Biol. 2020 Jan;28:101355. doi: 10.1016/j.redox.2019.101355. Epub 2019 Oct 23. Redox Biol. 2020. PMID: 31677554 Free PMC article.
-
Hydroxy-Safflower Yellow A Mitigates Vascular Remodeling in Rat Pulmonary Arterial Hypertension.Drug Des Devel Ther. 2024 Feb 20;18:475-491. doi: 10.2147/DDDT.S439686. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38405578 Free PMC article.
-
3'UTR shortening of HAS2 promotes hyaluronan hyper-synthesis and bioenergetic dysfunction in pulmonary hypertension.Matrix Biol. 2022 Aug;111:53-75. doi: 10.1016/j.matbio.2022.06.001. Epub 2022 Jun 4. Matrix Biol. 2022. PMID: 35671866 Free PMC article.
-
Cited2 inhibited hypoxia-induced proliferation and migration of PASMCs via the TGF-β1/Cited2/PPARγ pathway.Iran J Basic Med Sci. 2024;27(4):509-517. doi: 10.22038/IJBMS.2023.74455.16178. Iran J Basic Med Sci. 2024. PMID: 38419888 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

