CLIC4/Arf6 Pathway
- PMID: 30582444
- PMCID: PMC6325770
- DOI: 10.1161/CIRCRESAHA.118.313705
CLIC4/Arf6 Pathway
Abstract
Rationale: Increased expression of CLIC4 (chloride intracellular channel 4) is a feature of endothelial dysfunction in pulmonary arterial hypertension, but its role in disease pathology is not fully understood.
Objective: To identify CLIC4 effectors and evaluate strategies targeting CLIC4 signaling in pulmonary hypertension.
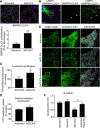
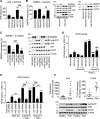
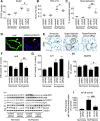
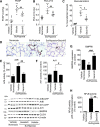
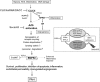
Methods and results: Proteomic analysis of CLIC4-interacting proteins in human pulmonary artery endothelial cells identified regulators of endosomal trafficking, including Arf6 (ADP ribosylation factor 6) GTPase activating proteins and clathrin, while CLIC4 overexpression affected protein regulators of vesicular trafficking, lysosomal function, and inflammation. CLIC4 reduced BMPRII (bone morphogenetic protein receptor II) expression and signaling as a result of Arf6-mediated reduction in gyrating clathrin and increased lysosomal targeting of the receptor. BMPRII expression was restored by Arf6 siRNA, Arf inhibitor Sec7 inhibitor H3 (SecinH3), and inhibitors of clathrin-mediated endocytosis but was unaffected by chloride channel inhibitor, indanyloxyacetic acid 94 or Arf1 siRNA. The effects of CLIC4 on NF-κB (nuclear factor-kappa B), HIF (hypoxia-inducible factor), and angiogenic response were prevented by Arf6 siRNA and SecinH3. Sugen/hypoxia mice and monocrotaline rats showed elevated expression of CLIC4, activation of Arf6 and NF-κB, and reduced expression of BMPRII in the lung. These changes were established early during disease development. Lung endothelium-targeted delivery of CLIC4 siRNA or treatment with SecinH3 attenuated the disease, reduced CLIC4/Arf activation, and restored BMPRII expression in the lung. Endothelial colony-forming cells from idiopathic pulmonary hypertensive patients showed upregulation of CLIC4 expression and Arf6 activity, suggesting potential importance of this pathway in the human condition.
Conclusions: Arf6 is a novel effector of CLIC4 and a new therapeutic target in pulmonary hypertension.
Keywords: chloride channels; endocytosis; endothelial cells; endothelial progenitor cells; hypertension, pulmonary.
Figures


Comment in
-
In Search of the Second Hit in Pulmonary Arterial Hypertension.Circ Res. 2019 Jan 4;124(1):6-8. doi: 10.1161/CIRCRESAHA.118.314270. Circ Res. 2019. PMID: 30605416 Free PMC article. No abstract available.
References
-
- Durrington HJ, Upton PD, Hoer S, Boname J, Dunmore BJ, Yang J, Crilley TK, Butler LM, Blackbourn DJ, Nash GB, Lehner PJ, Morrell NW. Identification of a lysosomal pathway regulating degradation of the bone morphogenetic protein receptor type II. J Biol Chem. 2010;285:37641–37649. doi: 10.1074/jbc.M110.132415. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

