A Dual Role of Heme Oxygenase-1 in Cancer Cells
- PMID: 30583467
- PMCID: PMC6337503
- DOI: 10.3390/ijms20010039
A Dual Role of Heme Oxygenase-1 in Cancer Cells
Abstract
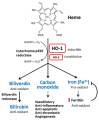
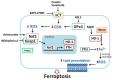
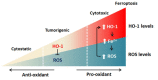
Heme oxygenase (HO)-1 is known to metabolize heme into biliverdin/bilirubin, carbon monoxide, and ferrous iron, and it has been suggested to demonstrate cytoprotective effects against various stress-related conditions. HO-1 is commonly regarded as a survival molecule, exerting an important role in cancer progression and its inhibition is considered beneficial in a number of cancers. However, increasing studies have shown a dark side of HO-1, in which HO-1 acts as a critical mediator in ferroptosis induction and plays a causative factor for the progression of several diseases. Ferroptosis is a newly identified iron- and lipid peroxidation-dependent cell death. The critical role of HO-1 in heme metabolism makes it an important candidate to mediate protective or detrimental effects via ferroptosis induction. This review summarizes the current understanding on the regulatory mechanisms of HO-1 in ferroptosis. The amount of cellular iron and reactive oxygen species (ROS) is the determinative momentum for the role of HO-1, in which excessive cellular iron and ROS tend to enforce HO-1 from a protective role to a perpetrator. Despite the dark side that is related to cell death, there is a prospective application of HO-1 to mediate ferroptosis for cancer therapy as a chemotherapeutic strategy against tumors.
Keywords: chemotherapy; ferroptosis; glutathione; heme oxygenase-1; iron; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

