Attenuation and Stability of CHIKV-NoLS, a Live-Attenuated Chikungunya Virus Vaccine Candidate
- PMID: 30583514
- PMCID: PMC6465992
- DOI: 10.3390/vaccines7010002
Attenuation and Stability of CHIKV-NoLS, a Live-Attenuated Chikungunya Virus Vaccine Candidate
Abstract
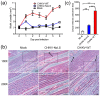
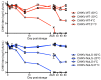
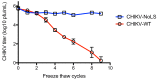
Our previous investigation of the nucleolar localisation sequence (NoLS) of chikungunya virus (CHIKV) capsid protein demonstrated the role of capsid in CHIKV virulence. Mutating the NoLS of capsid in CHIKV led to the development of a unique live-attenuated CHIKV vaccine candidate, termed CHIKV-NoLS. CHIKV-NoLS-immunised mice developed long-term immunity from CHIKV infection after a single dose. To further evaluate CHIKV-NoLS attenuation and suitability as a vaccine, we examined the footpad of inoculated mice for underlying CHIKV-NoLS-induced immunopathology by histological and flow cytometric analysis. In comparison to CHIKV-WT-infected mice, CHIKV-NoLS-inoculated mice exhibited minimal inflammation and tissue damage. To examine the stability of attenuation, the plaque phenotype and replication kinetics of CHIKV-NoLS were determined following extended in vitro passage. The average plaque size of CHIKV-NoLS remained notably smaller than CHIKV-WT after extended passage and attenuated replication was maintained. To examine thermostability, CHIKV-NoLS was stored at 21 °C, 4 °C, -20 °C and -80 °C and infectious CHIKV-NoLS quantified up to 84 days. The infectious titre of CHIKV-NoLS remains stable after 56 days when stored at either -20 °C or -80 °C. Interestingly, unlike CHIKV-WT, the infectious titre of CHIKV-NoLS is not sensitive to freeze thaw cycles. These data further demonstrate preclinical safety and stability of CHIKV-NoLS.
Keywords: alphavirus; chikungunya virus; live-attenuated; preclinical; vaccine.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures



References
-
- Sissoko D., Malvy D., Ezzedine K., Renault P., Moscetti F., Ledrans M., Pierre V. Post-epidemic chikungunya disease on reunion island: Course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl. Trop. Dis. 2009;3:e389. doi: 10.1371/journal.pntd.0000389. - DOI - PMC - PubMed
-
- Lemant J., Boisson V., Winer A., Thibault L., Andre H., Tixier F., Lemercier M., Antok E., Cresta M.P., Grivard P., et al. Serious acute chikungunya virus infection requiring intensive care during the reunion island outbreak in 2005-2006. Crit. Care Med. 2008;36:2536–2541. doi: 10.1097/CCM.0b013e318183f2d2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

