Optimizing Detection of Kidney Transplant Injury by Assessment of Donor-Derived Cell-Free DNA via Massively Multiplex PCR
- PMID: 30583588
- PMCID: PMC6352163
- DOI: 10.3390/jcm8010019
Optimizing Detection of Kidney Transplant Injury by Assessment of Donor-Derived Cell-Free DNA via Massively Multiplex PCR
Abstract
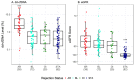
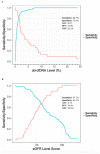
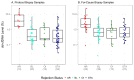
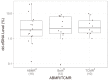
Standard noninvasive methods for detecting renal allograft rejection and injury have poor sensitivity and specificity. Plasma donor-derived cell-free DNA (dd-cfDNA) has been reported to accurately detect allograft rejection and injury in transplant recipients and shown to discriminate rejection from stable organ function in kidney transplant recipients. This study used a novel single nucleotide polymorphism (SNP)-based massively multiplexed PCR (mmPCR) methodology to measure dd-cfDNA in various types of renal transplant recipients for the detection of allograft rejection/injury without prior knowledge of donor genotypes. A total of 300 plasma samples (217 biopsy-matched: 38 with active rejection (AR), 72 borderline rejection (BL), 82 with stable allografts (STA), and 25 with other injury (OI)) were collected from 193 unique renal transplant patients; dd- cfDNA was processed by mmPCR targeting 13,392 SNPs. Median dd-cfDNA was significantly higher in samples with biopsy-proven AR (2.3%) versus BL (0.6%), OI (0.7%), and STA (0.4%) (p < 0.0001 all comparisons). The SNP-based dd-cfDNA assay discriminated active from non-rejection status with an area under the curve (AUC) of 0.87, 88.7% sensitivity (95% CI, 77.7⁻99.8%) and 72.6% specificity (95% CI, 65.4⁻79.8%) at a prespecified cutoff (>1% dd-cfDNA). Of 13 patients with AR findings at a routine protocol biopsy six-months post transplantation, 12 (92%) were detected positive by dd-cfDNA. This SNP-based dd-cfDNA assay detected allograft rejection with superior performance compared with the current standard of care. These data support the feasibility of using this assay to detect disease prior to renal failure and optimize patient management in the case of allograft injury.
Keywords: cfDNA; kidney transplantation; rejection.
Conflict of interest statement
T.K.S., J.L., I.D., P.T., R.D.S., C.C.-O., S.-C.H, and M.M.S. declare no conflicts. F.A.A., T.C., S.A.P., S.N., E.K., Z.P.D., A.R., S.S., B.Z., P.R.B., and S.M. are or were employees of Natera, Inc. with stock/options to own stock in the company.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

