Venom Proteome of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia: Toxicity Correlation, Immunoprofiling and Cross-Neutralization by Sea Snake Antivenom
- PMID: 30583590
- PMCID: PMC6356285
- DOI: 10.3390/toxins11010003
Venom Proteome of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia: Toxicity Correlation, Immunoprofiling and Cross-Neutralization by Sea Snake Antivenom
Abstract
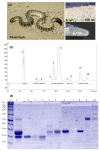
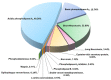
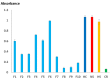
The venom proteome of Hydrophis curtus (synonym: Lapemis hardwickii) from Penang, Malaysia was investigated with nano-electrospray ionization-liquid chromatography tandem mass spectrometry (ESI-LCMS/MS) of the reverse-phase high-performance liquid chromatography (HPLC) venom fractions. Thirty distinct protein forms were identified as toxins from ten families. The three major protein families were phospholipase A₂ (PLA₂, 62.0% of total venom proteins), three-finger toxin (3FTX, 26.33%) and cysteine-rich secretory protein (CRiSP, 9.00%). PLA₂ comprises diverse homologues (11 forms), predominantly the acidic subtypes (48.26%). 3FTX composed of one short alpha-neurotoxin (SNTX, 22.89%) and four long alpha-neurotoxins (LNTX, 3.44%). Both SNTX and LNTX were lethal in mice (intravenous LD50 = 0.10 and 0.24 μg/g, respectively) but the PLA₂ were non-lethal (LD50 >1 μg/g). The more abundant and toxic SNTX appeared to be the main driver of venom lethality (holovenom LD50 = 0.20 μg/g). The heterologous Sea Snake Antivenom (SSAV, Australia) effectively cross-neutralized the venom (normalized potency = 9.35 mg venom neutralized per g antivenom) and the two neurotoxins in vivo, with the LNTX being neutralized more effectively (normalized potency = 3.5 mg toxin/g antivenom) than SNTX (normalized potency = 1.57 mg/g). SSAV immunorecognition was strong toward PLA₂ but moderate-to-weak toward the alpha-neurotoxins, indicating that neutralization of the alpha-neurotoxins should be further improved.
Keywords: Lapemis hardwickii; alpha-neurotoxins; envenomation; immunoreactivity; neutralization; phospholipase A2; three-finger toxins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Venom proteome of the yellow-lipped sea krait, Laticauda colubrina from Bali: Insights into subvenomic diversity, venom antigenicity and cross-neutralization by antivenom.J Proteomics. 2017 Aug 23;166:48-58. doi: 10.1016/j.jprot.2017.07.002. Epub 2017 Jul 5. J Proteomics. 2017. PMID: 28688916
-
De Novo Venom-Gland Transcriptomics of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia-Next-Generation Sequencing, Functional Annotation and Toxinological Correlation.Toxins (Basel). 2021 Feb 9;13(2):127. doi: 10.3390/toxins13020127. Toxins (Basel). 2021. PMID: 33572266 Free PMC article.
-
Venomics of Naja sputatrix, the Javan spitting cobra: A short neurotoxin-driven venom needing improved antivenom neutralization.J Proteomics. 2017 Mar 22;157:18-32. doi: 10.1016/j.jprot.2017.01.018. Epub 2017 Jan 31. J Proteomics. 2017. PMID: 28159706
-
A current perspective on snake venom composition and constituent protein families.Arch Toxicol. 2023 Jan;97(1):133-153. doi: 10.1007/s00204-022-03420-0. Epub 2022 Nov 27. Arch Toxicol. 2023. PMID: 36437303 Review.
-
Snake Venom PLA2, a Promising Target for Broad-Spectrum Antivenom Drug Development.Biomed Res Int. 2017;2017:6592820. doi: 10.1155/2017/6592820. Epub 2017 Nov 29. Biomed Res Int. 2017. PMID: 29318152 Free PMC article. Review.
Cited by
-
Snake Venom Proteomics of Samar Cobra (Naja samarensis) from the Southern Philippines: Short Alpha-Neurotoxins as the Dominant Lethal Component Weakly Cross-Neutralized by the Philippine Cobra Antivenom.Front Pharmacol. 2021 Dec 24;12:727756. doi: 10.3389/fphar.2021.727756. eCollection 2021. Front Pharmacol. 2021. PMID: 35002690 Free PMC article.
-
Antivenom preclinical efficacy testing against Asian snakes and their availability in Asia: A systematic review.PLoS One. 2023 Jul 19;18(7):e0288723. doi: 10.1371/journal.pone.0288723. eCollection 2023. PLoS One. 2023. PMID: 37467278 Free PMC article.
-
A Decoy-Receptor Approach Using Nicotinic Acetylcholine Receptor Mimics Reveals Their Potential as Novel Therapeutics Against Neurotoxic Snakebite.Front Pharmacol. 2019 Jul 30;10:848. doi: 10.3389/fphar.2019.00848. eCollection 2019. Front Pharmacol. 2019. PMID: 31417406 Free PMC article.
-
Convergent evolution of pain-inducing defensive venom components in spitting cobras.Science. 2021 Jan 22;371(6527):386-390. doi: 10.1126/science.abb9303. Science. 2021. PMID: 33479150 Free PMC article.
-
In Vitro neurotoxicity and myotoxicity of Malaysian Naja sumatrana and Naja kaouthia venoms: Neutralization by monovalent and Neuro Polyvalent Antivenoms from Thailand.PLoS One. 2022 Sep 12;17(9):e0274488. doi: 10.1371/journal.pone.0274488. eCollection 2022. PLoS One. 2022. PMID: 36094937 Free PMC article.
References
-
- Reid H.A. Epidemiology of sea-snake bites. J. Trop. Med. Hyg. 1975;78:106–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

