Epibatidine: A Promising Natural Alkaloid in Health
- PMID: 30583611
- PMCID: PMC6359223
- DOI: 10.3390/biom9010006
Epibatidine: A Promising Natural Alkaloid in Health
Abstract
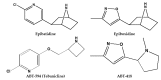
Epibatidine is a natural alkaloid that acts at nicotinic acetylcholine receptors (nAChRs). The present review aims to carefully discuss the affinity of epibatidine and its synthetic derivatives, analogues to nAChRs for α4β2 subtype, pharmacokinetic parameters, and its role in health. Published literature shows a low affinity and lack of binding of epibatidine and its synthetic analogues to plasma proteins, indicating their availability for metabolism. Because of its high toxicity, the therapeutic use of epibatidine is hampered. However, new synthetic analogs endowed from this molecule have been developed, with a better therapeutic window and improved selectivity. All these aspects are also discussed here. On the other hand, many reports are devoted to structure⁻activity relationships to obtain optically active epibatidine and its analogues, and to access its pharmacological effects. Although pharmacological results are obtained from experimental studies and only a few clinical trials, new perspectives are open for the discovery of new drug therapies.
Keywords: ABT-418; ABT-594; analgesics; epibatidine; nicotinic acetylcholine receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Spande T.F., Garraffo H.M., Edwards M.W., Yeh H.J.C., Pannell L., Daly J.W. Epibatidine: A novel (chloropyridyl)azabicycloheptane with potent analgesic activity from an Ecuadoran poison frog. J. Am. Chem. Soc. 1992:3475. doi: 10.1021/ja00035a048. - DOI
-
- Boyle J. Molecular biology of the cell, by b. Alberts, a. Johnson, j. Lewis, m. Raff, k. Roberts, and p. Walter. Biochem. Mol. Biol. Educ. 2008;36:317–318. doi: 10.1002/bmb.20192. - DOI
-
- Lloyd G.K., Williams M. Neuronal nicotinic acetylcholine receptors as novel drug targets. J. Pharmacol. Exp. Ther. 2000;292:461–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

