Cytoplasmic incompatibility management to support Incompatible Insect Technique against Aedes albopictus
- PMID: 30583743
- PMCID: PMC6304776
- DOI: 10.1186/s13071-018-3208-7
Cytoplasmic incompatibility management to support Incompatible Insect Technique against Aedes albopictus
Abstract
Background: The transinfection of the endosymbiotic bacterium Wolbachia provides a method to produce functionally sterile males to be used to suppress mosquito vectors. ARwP is a wPip Wolbachia infected Aedes albopictus which exhibits a bidirectional incompatibility pattern with wild-types. We coupled a modelistic approach with laboratory experiments to test ARwP as a control tool and evaluate the possible occurrence of population replacement following the release of ARwP females in a wild-type (SANG) population. Repeated male-only releases were simulated and tested in the laboratory in comparison with releases contaminated with 1% ARwP females. Model simulations also investigated how migration affects the outcome of contaminated releases. Finally, the mean level of egg fertility and the long-term evolution of populations constituted by two Wolbachia infection types were studied by testing SANG and ARwP Ae. albopictus and performing more general model simulations.
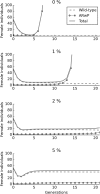
Results: The model was parametrized with laboratory data and simulations were compared with results of biological trials. Small populations of ARwP males and females were theoretically and experimentally demonstrated to rapidly become extinct when released in larger SANG populations. Male-only releases at a 5:1 ratio with respect to the wild-type males led to a complete suppression of the SANG population in a few generations. Contaminated releases were efficient as well but led to population replacement in the long term, when the wild-type population approached eradication. Migration significantly contrasted this trend as a 5% population turnover was sufficient to avoid any risk of population replacement. At equal frequencies between ARwP and SANG individuals, the mean egg fertility of the overall population was more than halved and suppression was self-sustaining until one of the two infection types extinguished.
Conclusions: In the case of bidirectional incompatibility patterns, the repeated release of incompatible males with small percentages of contaminant females could lead to population replacement in confined environments while it could be managed to target high efficiency and sustainability in wild-type suppression when systems are open to migration. This possibility is discussed based on various contexts and taking into consideration the possibility of integration with other control methods such as SIT and the use of larvicides.
Keywords: ARwP; SIT; Wolbachia; bidirectional incompatibility; population replacement; population suppression; risk assessment.
Conflict of interest statement
Ethics approval and consent to participate
Research carried out on invertebrates such as mosquitoes do not require a specific permit according to the directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes. The blood used for blood-feeding during the experiments was gently provided by Arianna Puggioli and Romeo Bellini at Centro Agricoltura e Ambiente (CAA, Crevalcore, BO, Italy) after collection in Camposanto (BO, Italy) during routine slaughtering of pigs in a national authorized abattoir (Az. Agr. All. Rubizzani CE IT N2L7D) at the highest possible standards strictly following EU laws and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Whitehorn J, Thi D, Kien H, Nguyen NM, Nguyen HL, Kyrylos PP, et al. Comparative susceptibility of Aedes albopictus and Aedes aegypti to dengue virus infection after feeding on blood of viremic humans: implications for public health. J Infect Dis. 2015;212:1182–1190. doi: 10.1093/infdis/jiv173. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

