Two-step pathway for isoprenoid synthesis
- PMID: 30584096
- PMCID: PMC6329939
- DOI: 10.1073/pnas.1812935116
Two-step pathway for isoprenoid synthesis
Abstract
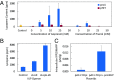
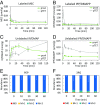
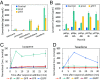
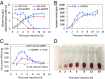
Isoprenoids comprise a large class of chemicals of significant interest due to their diverse properties. Biological production of isoprenoids is considered to be the most efficient way for their large-scale production. Isoprenoid biosynthesis has thus far been dependent on pathways inextricably linked to glucose metabolism. These pathways suffer from inherent limitations due to their length, complex regulation, and extensive cofactor requirements. Here, we present a synthetic isoprenoid pathway that aims to overcome these limitations. This isopentenol utilization pathway (IUP) can produce isopentenyl diphosphate or dimethylallyl diphosphate, the main precursors to isoprenoid synthesis, through sequential phosphorylation of isopentenol isomers isoprenol or prenol. After identifying suitable enzymes and constructing the pathway, we attempted to probe the limits of the IUP for producing various isoprenoid downstream products. The IUP flux exceeded the capacity of almost all downstream pathways tested and was competitive with the highest isoprenoid fluxes reported.
Keywords: biosynthesis; isopentenol; isoprenoids; pathway; utilization.
Conflict of interest statement
Conflict of interest statement: The authors are listed as inventors in a pending patent application (no. US 62/677,421; applicant, Massachusetts Institute of Technology) encompassing all the information presented here, as well as additional applications.
Figures


References
-
- Chandran SS, Kealey JT, Reeves CD. Microbial production of isoprenoids. Process Biochem. 2011;46:1703–1710.
-
- Kirby J, Keasling JD. Biosynthesis of plant isoprenoids: Perspectives for microbial engineering. Annu Rev Plant Biol. 2009;60:335–355. - PubMed
-
- Nagegowda DA. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010;584:2965–2973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

