Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/Smad signaling pathway
- PMID: 30584275
- PMCID: PMC6284527
- DOI: 10.2147/DDDT.S186726
Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/Smad signaling pathway
Erratum in
-
Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/Smad signaling pathway [Corrigendum].Drug Des Devel Ther. 2019 May 24;13:1819. doi: 10.2147/DDDT.S215949. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31213770 Free PMC article. No abstract available.
Abstract
Purpose: Liver fibrosis is a worldwide health issue. Development of effective new drugs for treatment of this disease is of great importance. This study investigated the therapeutic effects of ferulic acid on liver fibrosis in vitro and in vivo.
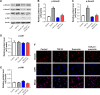
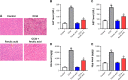
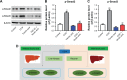
Materials and methods: Human hepatic stellate cell line (HSC) LX-2 was used for in vitro assays. Transforming growth factor β1 (TGF-β1) was used to induce hepatic fibrosis in LX-2 cells. Western blot was used to detect protein levels of collagen I, fibronectin, α-smooth muscle actin (SMA), p-Smad2, p-Smad3, p-p38, and p-JNK. Gene expression was measured by RT-qPCR. Fluorescence staining was used to determine localization of Smad4. CCl4-induced hepatic fibrosis in SD rats was used as an in vivo model. Histological features were detected by hematoxylin and eosin staining. Levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), hexadecenoic acid (HA), and hydroxyproline (Hyp) were measured by ELISA.
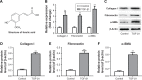
Results: TGF-β1 treatment significantly increased levels of collagen I, fibronectin, α-SMA, p-Smad2, p-Smad3, and Smad4 in LX-2 cells. Ferulic acid improved TGF-β1-induced hepatic fibrosis via regulation of the TGF-β1/Smad pathway. Consistent with in vitro data, CCl4 caused severe hepatic fibrosis in SD rats, as determined by ALT, AST, HA, and Hyp upregulation. Protein levels of p-Smad2 and p-Smad3 in liver tissues were significantly increased following treatment with CCl4. All CCL4-induced changes were markedly attenuated by ferulic acid treatment.
Conclusion: Ferulic acid potently improved hepatic fibrosis via inhibition of the TGF-β1/Smad pathway in vitro and in vivo. These findings provided evidence for potential use of ferulic acid to treat or prevent liver fibrosis.
Keywords: CCl4; Smad signaling pathway; TGF-β1; ferulic acid; hepatic fibrosis.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Friedman SL. Liver fibrosis – from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. - PubMed
-
- Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21(3):397–416. - PubMed
-
- Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol. 2005;45:605–628. - PubMed
-
- Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275(4):2247–2250. - PubMed
-
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

