Indacaterol acetate/mometasone furoate provides sustained improvements in lung function compared with salmeterol xinafoate/fluticasone propionate in patients with moderate-to-very-severe COPD: results from a Phase II randomized, double-blind 12-week study
- PMID: 30584293
- PMCID: PMC6287650
- DOI: 10.2147/COPD.S179293
Indacaterol acetate/mometasone furoate provides sustained improvements in lung function compared with salmeterol xinafoate/fluticasone propionate in patients with moderate-to-very-severe COPD: results from a Phase II randomized, double-blind 12-week study
Abstract
Background and purpose: Fixed-dose combinations of a long-acting beta agonist and an inhaled corticosteroid are more effective than the individual components in COPD. The primary study objective was to demonstrate that the combination indacaterol acetate/mometasone furoate (IND/MF [QMF149]) was non-inferior to the twice-daily combination salmeterol xinafoate/fluticasone propionate (Sal/Flu) in terms of trough FEV1 at week 12 (day 85). Secondary objectives were to compare the efficacy of IND/MF (QMF149) vs Sal/Flu with respect to other lung function parameters, COPD exacerbations, symptoms and dyspnea, health status/health-related quality of life, and rescue medication use.
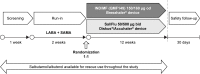
Materials and methods: This was a 12-week multicenter, randomized, double-blind, double-dummy, parallel-group, Phase II study in patients with moderate-to-very-severe COPD, who were randomized (1:1) to IND/MF (QMF149) (150/160 µg once daily; n=316) or Sal/Flu (50/500 µg twice daily; n=313).
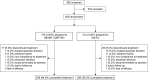
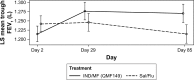
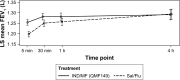
Results: Over 90% of patients completed the study: 94.6% in the IND/MF (QMF149) group and 92.0% in the Sal/Flu group. The primary objective of non-inferiority of IND/MF (QMF149) to Sal/Flu for trough FEV1 at week 12 (day 85) was met: the lower limit of the CI (95% CI: 27.7, 83.3 mL) was greater than -60 mL. The analysis for superiority of IND/MF (QMF149) to Sal/Flu demonstrated superiority of IND/MF (QMF149), with a difference of 56 mL (P<0.001). In addition, IND/MF (QMF149) treatment significantly improved COPD exacerbation-related parameters during the 12-week period. Other significant improvements with IND/MF (QMF 149) vs Sal/Flu were noted for dyspnea at week 12 and other COPD symptoms and COPD rescue medication use over the 12 weeks. The safety and tolerability profiles of both the treatments were similar.
Conclusion: IND/MF (QMF149) (150/160 µg once daily) offered superior lung function and symptom efficacy and a favorable safety profile compared with Sal/Flu (50/500 µg twice daily) in patients with moderate-to-very severe COPD.
Keywords: COPD; LABA/ICS combinations; fixed-combination inhalers; indacaterol; mometasone; once-daily inhalers.
Conflict of interest statement
Disclosure AMT, BH and AR are employees of Novartis Pharma AG. WC is an employee of Novartis Pharmaceuticals Corporation. KMB declares that no personal payments were received from any pharmaceutical entity in the past 5 years. KMB and JB are full-time employees of Insaf Respiratory Research Institute. AMK is an employee of the Pulmonary Research Institute at LungClinic Grosshansdorf. AMK received speaking honoraria, honoraria for participation in advisory board meetings, and travel support for attending congresses in respiratory medicine from Boehringer Ingelheim, AstraZeneca, and Novartis. RNVZS has received honoraria for academic work from Novartis, AZ, CIPLA, ASPEN, Pfizer, GSK, and MSD. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Once-daily mometasone plus indacaterol versus mometasone or twice-daily fluticasone plus salmeterol in patients with inadequately controlled asthma (PALLADIUM): a randomised, double-blind, triple-dummy, controlled phase 3 study.Lancet Respir Med. 2020 Oct;8(10):987-999. doi: 10.1016/S2213-2600(20)30178-8. Epub 2020 Jul 9. Lancet Respir Med. 2020. PMID: 32653075 Clinical Trial.
-
Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study.Lancet Respir Med. 2020 Oct;8(10):1000-1012. doi: 10.1016/S2213-2600(20)30190-9. Epub 2020 Jul 9. Lancet Respir Med. 2020. PMID: 32653074 Clinical Trial.
-
Fixed-dose combination of indacaterol/glycopyrronium/mometasone furoate once-daily versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma: A randomised, Phase IIIb, non-inferiority study (ARGON).Respir Med. 2020 Aug-Sep;170:106021. doi: 10.1016/j.rmed.2020.106021. Epub 2020 May 27. Respir Med. 2020. PMID: 32843164 Clinical Trial.
-
A Review of the Unique Drug Development Strategy of Indacaterol Acetate/Glycopyrronium Bromide/Mometasone Furoate: A First-in-Class, Once-Daily, Single-Inhaler, Fixed-Dose Combination Treatment for Asthma.Adv Ther. 2022 Jun;39(6):2365-2378. doi: 10.1007/s12325-021-02025-w. Epub 2022 Jan 24. Adv Ther. 2022. PMID: 35072888 Free PMC article. Review.
-
Mometasone/Indacaterol/Glycopyrronium (MF/IND/GLY) and MF/IND at Different MF Strengths versus Fluticasone Propionate/Salmeterol Xinafoate (FLU/SAL) and FLU/SAL+ Tiotropium in Patients with Asthma.J Asthma Allergy. 2023 Jan 20;16:123-134. doi: 10.2147/JAA.S392975. eCollection 2023. J Asthma Allergy. 2023. PMID: 36714049 Free PMC article. Review.
Cited by
-
Optimizing Spray-Dried Porous Particles for High Dose Delivery with a Portable Dry Powder Inhaler.Pharmaceutics. 2021 Sep 21;13(9):1528. doi: 10.3390/pharmaceutics13091528. Pharmaceutics. 2021. PMID: 34575603 Free PMC article. Review.
-
LABA/LAMA versus LABA/ICS fixed-dose combinations in the prevention of COPD exacerbations: a modeling analysis of literature aggregate data.Eur J Clin Pharmacol. 2023 Oct;79(10):1321-1332. doi: 10.1007/s00228-023-03543-y. Epub 2023 Jul 29. Eur J Clin Pharmacol. 2023. PMID: 37507595
References
-
- Collaborators GBDCRD. Soriano JB, Abajobir AA, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. - PMC - PubMed
-
- Hutchinson A, Brand C, Irving L, Roberts C, Thompson P, Campbell D. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Intern Med J. 2010;40(5):364–371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

