Mutation in POLR3K causes hypomyelinating leukodystrophy and abnormal ribosomal RNA regulation
- PMID: 30584594
- PMCID: PMC6283457
- DOI: 10.1212/NXG.0000000000000289
Mutation in POLR3K causes hypomyelinating leukodystrophy and abnormal ribosomal RNA regulation
Abstract
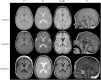
Objective: To identify the genetic cause of hypomyelinating leukodystrophy in 2 consanguineous families.
Methods: Homozygosity mapping combined with whole-exome sequencing of consanguineous families was performed. Mutation consequences were determined by studying the structural change of the protein and by the RNA analysis of patients' fibroblasts.
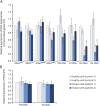
Results: We identified a biallelic mutation in a gene coding for a Pol III-specific subunit, POLR3K (c.121C>T/p.Arg41Trp), that cosegregates with the disease in 2 unrelated patients. Patients expressed neurologic and extraneurologic signs found in POLR3A- and POLR3B-related leukodystrophies with a peculiar severe digestive dysfunction. The mutation impaired the POLR3K-POLR3B interactions resulting in zebrafish in abnormal gut development. Functional studies in the 2 patients' fibroblasts revealed a severe decrease (60%-80%) in the expression of 5S and 7S ribosomal RNAs in comparison with control.
Conclusions: These analyses underlined the key role of ribosomal RNA regulation in the development and maintenance of the white matter and the cerebellum as already reported for diseases related to genes involved in transfer RNA or translation initiation factors.
Figures

References
-
- Dieci G, Fiorino G, Castelnuovo M, Teichmann M, Pagano A. The expanding RNA polymerase III transcriptome. Trends Genet 2007;23:614–622. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
