Identification of Hybrid Insulin Peptides (HIPs) in Mouse and Human Islets by Mass Spectrometry
- PMID: 30585061
- PMCID: PMC7597855
- DOI: 10.1021/acs.jproteome.8b00875
Identification of Hybrid Insulin Peptides (HIPs) in Mouse and Human Islets by Mass Spectrometry
Abstract
We recently discovered hybrid insulin peptides (HIPs) as a novel class of post-translationally modified peptides in murine-derived beta cell tumors, and we demonstrated that these molecules are autoantigens in type 1 diabetes (T1D). A HIP consists of an insulin fragment linked to another secretory granule peptide via a peptide bond. We verified that autoreactive CD4 T cells in both mouse and human autoimmune diabetes recognize these modified peptides. Here, we use mass spectrometric analyses to confirm the presence of HIPs in both mouse and human pancreatic islets. We also present criteria for the confident identification of these peptides. This work supports the hypothesis that HIPs are autoantigens in human T1D and provides a foundation for future efforts to interrogate this previously unknown component of the beta cell proteome.
Keywords: beta cell proteome; hybrid insulin peptide (HIP); mass spectrometry; pancreatic islets; type 1 diabetes (T1D).
Conflict of interest statement
The authors declare the following competing financial interest(s): A provisional patent application has been submitted for the use of hybrid insulin peptides and HIP-reactive T cells as biomarkers in T1D (TD, KH).
Figures


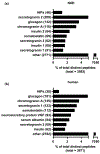
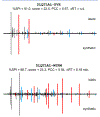
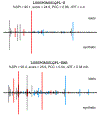
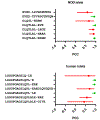
Similar articles
-
Characterization of Human CD4 T Cells Specific for a C-Peptide/C-Peptide Hybrid Insulin Peptide.Front Immunol. 2021 May 25;12:668680. doi: 10.3389/fimmu.2021.668680. eCollection 2021. Front Immunol. 2021. PMID: 34113344 Free PMC article.
-
HIPs and HIP-reactive T cells.Clin Exp Immunol. 2019 Dec;198(3):306-313. doi: 10.1111/cei.13335. Epub 2019 Jun 17. Clin Exp Immunol. 2019. PMID: 31132145 Free PMC article. Review.
-
Hybrid insulin peptide isomers spontaneously form in pancreatic beta-cells from an aspartic anhydride intermediate.J Biol Chem. 2023 Nov;299(11):105264. doi: 10.1016/j.jbc.2023.105264. Epub 2023 Sep 19. J Biol Chem. 2023. PMID: 37734557 Free PMC article.
-
Identifying New Hybrid Insulin Peptides (HIPs) in Type 1 Diabetes.Front Immunol. 2021 Apr 30;12:667870. doi: 10.3389/fimmu.2021.667870. eCollection 2021. Front Immunol. 2021. PMID: 33995402 Free PMC article. Review.
-
Mapping of a hybrid insulin peptide in the inflamed islet β-cells from NOD mice.Front Immunol. 2024 Feb 22;15:1348131. doi: 10.3389/fimmu.2024.1348131. eCollection 2024. Front Immunol. 2024. PMID: 38455055 Free PMC article.
Cited by
-
Understanding Islet Autoantibodies in Prediction of Type 1 Diabetes.J Endocr Soc. 2024 Jan 2;8(1):bvad160. doi: 10.1210/jendso/bvad160. eCollection 2023 Dec 1. J Endocr Soc. 2024. PMID: 38169963 Free PMC article. Review.
-
Novel T-Cell Reactivities to Hybrid Insulin Peptides in Islet Autoantibody-Positive At-Risk Individuals.Diabetes. 2025 Jun 1;74(6):933-942. doi: 10.2337/db24-0611. Diabetes. 2025. PMID: 39820647
-
MHC Class II Presentation in Autoimmunity.Cells. 2023 Jan 14;12(2):314. doi: 10.3390/cells12020314. Cells. 2023. PMID: 36672249 Free PMC article. Review.
-
Characterization of Human CD4 T Cells Specific for a C-Peptide/C-Peptide Hybrid Insulin Peptide.Front Immunol. 2021 May 25;12:668680. doi: 10.3389/fimmu.2021.668680. eCollection 2021. Front Immunol. 2021. PMID: 34113344 Free PMC article.
-
Antigen-specific T cell responses in autoimmune diabetes.Front Immunol. 2024 Aug 15;15:1440045. doi: 10.3389/fimmu.2024.1440045. eCollection 2024. Front Immunol. 2024. PMID: 39211046 Free PMC article. Review.
References
-
- Delong T; Wiles TA; Baker RL; Bradley B; Barbour G; Reisdorph R; Armstrong M; Powell RL; Reisdorph N; Kumar N; Elso CM; DeNicola M; Bottino R; Powers AC; Harlan DM; Kent SC; Mannering SI; Haskins K. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 2016, 351 (6274), 711–4. - PMC - PubMed
-
- Babon JA; DeNicola ME; Blodgett DM; Crevecoeur I; Buttrick TS; Maehr R; Bottino R; Naji A; Kaddis J; Elyaman W; James EA; Haliyur R; Brissova M; Overbergh L; Mathieu C; Delong T; Haskins K; Pugliese A; Campbell-Thompson M; Mathews C; Atkinson MA; Powers AC; Harlan DM; Kent SC Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat. Med. 2016, 22 (12), 1482–1487. - PMC - PubMed
-
- Ito Y; Ashenberg O; Pyrdol J; Luoma AM; Rozenblatt-Rosen O; Hofree M; Christian E; Ferrari de Andrade L; Tay RE; Teyton L; Regev A; Dougan SK; Wucherpfennig KW Rapid CLIP dissociation from MHC II promotes an unusual antigen presentation pathway in autoimmunity. J. Exp. Med. 2018, 215 (10), 2617–2635. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

