Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System
- PMID: 30585199
- PMCID: PMC6356784
- DOI: 10.3390/v11010010
Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System
Abstract
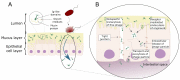
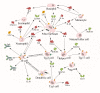
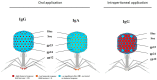
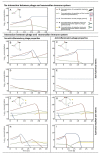
The human body is host to large numbers of bacteriophages (phages)⁻a diverse group of bacterial viruses that infect bacteria. Phage were previously regarded as bystanders that only impacted immunity indirectly via effects on the mammalian microbiome. However, it has become clear that phages also impact immunity directly, in ways that are typically anti-inflammatory. Phages can modulate innate immunity via phagocytosis and cytokine responses, but also impact adaptive immunity via effects on antibody production and effector polarization. Phages may thereby have profound effects on the outcome of bacterial infections by modulating the immune response. In this review we highlight the diverse ways in which phages interact with human cells. We present a computational model for predicting these complex and dynamic interactions. These models predict that the phageome may play important roles in shaping mammalian-bacterial interactions.
Keywords: adaptive immunity; bacteriophage; human host; immunology; innate immunity; phage-human host interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Proctor L., Sechi S., Di Giacomo N., Fettweis J., Jefferson K., Strauss J., III, Rubens C., Brooks J., Girerd P., Huang B., et al. The Integrative Human Microbiome Project: Dynamic Analysis of Microbiome-Host Omics Profiles during Periods of Human Health and Disease. Cell Host Microbe. 2014;16:276–289. doi: 10.1016/j.chom.2014.08.014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

