What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies
- PMID: 30585835
- PMCID: PMC6365258
- DOI: 10.1097/MBC.0000000000000789
What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies
Abstract
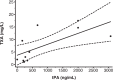
: Intravenous tranexamic acid (TXA) reduces death because of bleeding in patients with trauma and postpartum haemorrhage. However, in some settings intravenous injection is not feasible. To find different routes of administration, we first need to determine the minimal concentration of TXA in the blood that is required to inhibit fibrinolysis.We conducted a systematic review of in-vitro and in-vivo pharmacodynamics studies. We searched MEDLINE, EMBASE, OviSP, and ISI Web of Science from database inception to November 2017 for all in-vitro (including simulated clotting models) or in-vivo studies reporting the relationship between the TXA concentration in blood or plasma and any reliable measure of fibrinolysis.We found 21 studies of which 20 were in vitro and one was in vivo. Most in-vitro studies stimulated fibrinolysis with tissue plasminogen activator and measured fibrinolysis using viscoelastic, optical density, or immunological assays. TXA concentrations between 10 and 15 mg/l resulted in substantial inhibition of fibrinolysis, although concentrations between 5 and 10 mg/l were partly inhibitory.TXA concentrations of 10-15 mg/l may be suitable targets for pharmacokinetic studies, although TXA concentrations above 5 mg/l may also be effective.
Figures
Similar articles
-
Alternative routes to intravenous tranexamic acid for postpartum hemorrhage: A systematic search and narrative review.Int J Gynaecol Obstet. 2022 Jun;158 Suppl 1(Suppl 1):40-45. doi: 10.1002/ijgo.14201. Int J Gynaecol Obstet. 2022. PMID: 35762806 Free PMC article. Review.
-
Tranexamic acid mediates proinflammatory and anti-inflammatory signaling via complement C5a regulation in a plasminogen activator-dependent manner.J Trauma Acute Care Surg. 2019 Jan;86(1):101-107. doi: 10.1097/TA.0000000000002092. J Trauma Acute Care Surg. 2019. PMID: 30575685
-
Synergism of red blood cells and tranexamic acid in the inhibition of fibrinolysis.J Thromb Haemost. 2024 Mar;22(3):794-804. doi: 10.1016/j.jtha.2023.11.009. Epub 2023 Nov 26. J Thromb Haemost. 2024. PMID: 38016517
-
Effective tranexamic acid concentration for 95% inhibition of tissue-type plasminogen activator-induced hyperfibrinolysis in full-term pregnant women: a prospective interventional study.Blood Coagul Fibrinolysis. 2021 Apr 1;32(3):186-193. doi: 10.1097/MBC.0000000000001015. Blood Coagul Fibrinolysis. 2021. PMID: 33470644 Clinical Trial.
-
Tranexamic acid in trauma: how should we use it?J Thromb Haemost. 2015 Jun;13 Suppl 1:S195-9. doi: 10.1111/jth.12878. J Thromb Haemost. 2015. PMID: 26149023 Review.
Cited by
-
Functional Testing for Tranexamic Acid Duration of Action Using Modified Viscoelastometry.Transfus Med Hemother. 2021 Mar;48(2):109-117. doi: 10.1159/000511230. Epub 2020 Nov 9. Transfus Med Hemother. 2021. PMID: 33976611 Free PMC article.
-
Maintaining the balance: the critical role of plasmin activity in orthopedic surgery injury response.J Thromb Haemost. 2023 Oct;21(10):2653-2665. doi: 10.1016/j.jtha.2023.08.002. Epub 2023 Aug 8. J Thromb Haemost. 2023. PMID: 37558131 Free PMC article. Review.
-
Exploratory Randomised Trial of Tranexamic Acid to Decrease Postoperative Delirium in Adults Undergoing Lumbar Fusion: A trial stopped early.medRxiv [Preprint]. 2024 Oct 17:2024.10.16.24315638. doi: 10.1101/2024.10.16.24315638. medRxiv. 2024. Update in: BJA Open. 2025 Apr 14;14:100403. doi: 10.1016/j.bjao.2025.100403. PMID: 39484259 Free PMC article. Updated. Preprint.
-
Pharmacokinetics of Curative Tranexamic Acid in Parturients Undergoing Cesarean Delivery.Pharmaceutics. 2022 Mar 6;14(3):578. doi: 10.3390/pharmaceutics14030578. Pharmaceutics. 2022. PMID: 35335955 Free PMC article.
-
Tranexamic acid in a mouse model of cerebral amyloid angiopathy: setting the stage for a novel stroke treatment approach.Res Pract Thromb Haemost. 2023 Aug 9;7(6):102166. doi: 10.1016/j.rpth.2023.102166. eCollection 2023 Aug. Res Pract Thromb Haemost. 2023. PMID: 37694270 Free PMC article.
References
-
- McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs 2012; 72:585–617. - PubMed
-
- Okamoto S, Okamoto U. Amino-methyl-cyclohexane-carboxylic acid: AMCHA a new potent inhibitor of the fibrinolysis. Keio J Med 1962; 11:105–115.
-
- Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, et al. CRASH-2 Collaborators. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011; 377:1096–1101. - PubMed
-
- Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010; 376:23–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

