Menopausal hormone therapy trends before versus after 2002: impact of the Women's Health Initiative Study Results
- PMID: 30586004
- PMCID: PMC6538484
- DOI: 10.1097/GME.0000000000001282
Menopausal hormone therapy trends before versus after 2002: impact of the Women's Health Initiative Study Results
Abstract
Objective: To better understand how to educate patients and providers about study findings relevant to treatment guidelines, we assessed pre- versus post-Women's Health Initiative (WHI) differences in menopausal hormone therapy (MHT) initiation and continuation and their correlates, and in women's reasons for initiation and discontinuation.
Methods: We analyzed survey data from up to 14 approximately annual visits over 17 years (1996-2013) from 3,018 participants in the Study of Women's Health Across the Nation, a prospective cohort study. We used logistic regression to compare pre- versus post-WHI associations of covariates with MHT initiation and continuation, and to compare pre- versus post-WHI reasons for initiation and continuation.
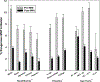
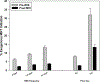
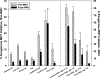
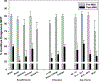
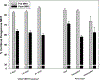
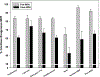
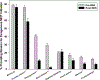
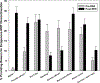
Results: MHT initiation dropped from 8.6% pre-WHI to 2.8% post-WHI (P < 0.0001), and the corresponding decrease in MHT continuation was 84.0% to 62.0% (P < 0.0001). Decreases in MHT initiation and continuation occurred across a range of participant subgroups, consistent with wide dissemination of post-WHI recommendations. However, contrary to current guidelines, we found large declines in MHT use in subgroups for whom MHT is often recommended, that is, younger women and those with more vasomotor symptoms. Post-WHI, women's reasons for MHT initiation and discontinuation reflected concerns highlighted by WHI results. The largest declines in initiation reasons were for reducing risks of osteoporosis and heart disease, whereas the largest increases in discontinuation reasons were for media reports and provider advice.
Conclusions: Immediate post-WHI recommendations for MHT use were widely adopted. MHT risks documented in older women, however, may have led younger symptomatic women to forgo MHT for symptom relief.
Conflict of interest statement
Potential conflicts of interest: No other potential conflicts of interest.
Figures
Comment in
-
A call to increase the use of hormone therapy to prevent disease in symptomatic postmenopausal women.Menopause. 2019 Mar 18;26(6):573-575. doi: 10.1097/GME.0000000000001324. Menopause. 2019. PMID: 30889088 No abstract available.
References
-
- Ettinger B, Wang SM, Leslie RS, et al. Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause 2012;19:610–615. - PubMed
-
- Steinkellner AR, Denison SE, Eldridge SL, Lenzi LL, Chen W, Bowlin SJ. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women’s Health Initiative. Menopause 2012;19:616–621. - PubMed
-
- Weissfeld JL, Liu W, Woods C, et al. Trends in oral and vaginally administered estrogen use among US women 50 years of age and older with commercial health insurance. Menopause 2018;25:611–614. - PubMed
-
- North American Menopause Society. The 2017 position statement of the North American Menopause Society. Menopause 2017;24;728–753. - PubMed
-
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015;100:3975–4011. - PubMed

