The IASP classification of chronic pain for ICD-11: chronic neuropathic pain
- PMID: 30586071
- PMCID: PMC6310153
- DOI: 10.1097/j.pain.0000000000001365
The IASP classification of chronic pain for ICD-11: chronic neuropathic pain
Abstract
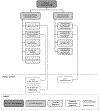
The upcoming 11th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD) of the World Health Organization (WHO) offers a unique opportunity to improve the representation of painful disorders. For this purpose, the International Association for the Study of Pain (IASP) has convened an interdisciplinary task force of pain specialists. Here, we present the case for a reclassification of nervous system lesions or diseases associated with persistent or recurrent pain for ≥3 months. The new classification lists the most common conditions of peripheral neuropathic pain: trigeminal neuralgia, peripheral nerve injury, painful polyneuropathy, postherpetic neuralgia, and painful radiculopathy. Conditions of central neuropathic pain include pain caused by spinal cord or brain injury, poststroke pain, and pain associated with multiple sclerosis. Diseases not explicitly mentioned in the classification are captured in residual categories of ICD-11. Conditions of chronic neuropathic pain are either insufficiently defined or missing in the current version of the ICD, despite their prevalence and clinical importance. We provide the short definitions of diagnostic entities for which we submitted more detailed content models to the WHO. Definitions and content models were established in collaboration with the Classification Committee of the IASP's Neuropathic Pain Special Interest Group (NeuPSIG). Up to 10% of the general population experience neuropathic pain. The majority of these patients do not receive satisfactory relief with existing treatments. A precise classification of chronic neuropathic pain in ICD-11 is necessary to document this public health need and the therapeutic challenges related to chronic neuropathic pain.
Conflict of interest statement
Conflict of interest statement
Joachim Scholz has received research support from the Thompson Family Foundation and Acetylon, and is now an employee of Biogen. This work was completed before he joined the company. Biogen did not have a role in the design, conduct, analysis, interpretation or funding of the research related to this work. Nanna B. Finnerup has received honoraria for serving on advisory boards or speaker panels from Teva, Novartis, Astellas, Grünenthal, Mitshubishe Tanabe, Novartis and Teva. Nadine Attal has received speaker fees from Pfizer and consultant fees from Aptinyx, Grünenthal, Mundipharma, Novartis, Sanofi Pasteur, and Teva. Ralf Baron reports receiving research grants from Genzyme, Grünenthal, Mundipharma and Pfizer, and honoraria for serving on advisory boards or speakers panels from Abbvie, Allergan, Astellas, AstraZeneca, Bayer, Biogen, Biotest Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Desitin, Eisai, Galapagos NV, Genentech, Genzyme, Glenmark, Grünenthal, Kyowa Kirin, Lilly, Medtronic, Merck MSD, Mundipharma, Novartis, Pfizer, Sanofi Pasteur, Seqirus, TAD, Teva, and Vertex. He is also member of the Innovative Medicines Initiative (IMI), a public-private partnership between the European Union and the European Federation of Pharmaceutical Industries and Associations (EFPIA). Giorgio Cruccu reports grants or personal fees from Alfa Sigma, Angelini, Biogen, Mundipharma, and Pfizer. Michael First reports personal fees from Lundbeck International Neuroscience Foundation, outside the submitted work. Maria Adele Giamberardino reports personal fees from IBSA Institute Biochimique, personal fees from EPITECH Group, personal fees from Helsinn Healthcare, grants from EPITECH Group, grants from Helsinn Healthcare, outside the submitted work. Stein Kaasa reports that he is Eir solution - stockholder. Turo Nurmikko has received personal fees from Abide, Astellas, Mundipharma and Nexstim, and research grants from the Pain Relief Foundation and the National Institute of Health Research in the United Kingdom. Srinivasa N. Raja has received research grants from Medtronic, and serves in the advisory boards of Allergan, Daiichi Sankyo, and Aptinyx. Andrew S.C. Rice has received research funding from Orion, has consulted or participated in advisory boards for Imperial College Consultants, including remunerated work for Abide, Astellas, Galapagos, Lateral, Peter McNaughton from King’s College London and Cambridge University, Merck, Mitsubishi, Novartis, Orion, Pharmaleads, Quartet, and Toray. He owned share options in Spinifex from which personal benefit accrued upon the acquisition of Spinifex by Novartis, and from which future milestone payments may occur. ASCR is also named inventor on patents WO 2005/079771, EP13702262.0, and WO2013/110945. Shuu-Jiun Wang reports personal fees from Eli-Lilly, personal fees from Daiichi-Sankyo, grants and personal fees from Pfizer, Taiwan, personal fees from Eisai, personal fees from Bayer, personal fees from Boehringer Ingelheim, outside the submitted work. Antonia Barke reports personal fees from IASP, during the conduct of the study. Winfried Rief reports grants from IASP, during the conduct of the study; personal fees from Heel, personal fees from Berlin Chemie, outside the submitted work. Rolf-Detlef Treede reports grants from Boehringer Ingelheim, Astellas, AbbVie, Bayer, personal fees from Astellas, Grünenthal, Bauerfeind, Hydra, Bayer, grants from EU, DFG, BMBF, outside the submitted work. The remaining authors have no conflicts of interest to declare.
Figures
References
-
- Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice AS, Segerdahl M, Serra J, Sindrup S, Sommer C, Tolle T, Vollert J, Treede RD. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain 2017;158:261–272. - PMC - PubMed
-
- Bennett* MI, Kaasa* S, Barke* A, Korwisi B, Rief W, Treede R-D, The IASP Taskforce for the Classification of Chronic Pain. The IASP Classification of Chronic Pain for ICD-11: Chronic cancer-related pain. Pain 2018. - PubMed
-
- Cavaletti G, Alberti P, Frigeni B, Piatti M, Susani E. Chemotherapy-induced neuropathy. Curr Treat Options Neurol 2011;13:180–190. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

