Insights into the role of endoplasmic reticulum stress in skin function and associated diseases
- PMID: 30586218
- PMCID: PMC6362265
- DOI: 10.1111/febs.14739
Insights into the role of endoplasmic reticulum stress in skin function and associated diseases
Abstract
Endoplasmic reticulum (ER) stress is a mechanism that allows the protection of normal cellular functions in response to both internal perturbations, such as accumulation of unfolded proteins, and external perturbations, for example redox stress, UVB irradiation, and infection. A hallmark of ER stress is the accumulation of misfolded and unfolded proteins. Physiological levels of ER stress trigger the unfolded protein response (UPR) that is required to restore normal ER functions. However, the UPR can also initiate a cell death program/apoptosis pathway in response to excessive or persistent ER stress. Recently, it has become evident that chronic ER stress occurs in several diseases, including skin diseases such as Darier's disease, rosacea, vitiligo and melanoma; furthermore, it is suggested that ER stress is directly involved in the pathogenesis of these disorders. Here, we review the role of ER stress in skin function, and discuss its significance in skin diseases.
Keywords: endoplasmic reticulum stress; skin disease; skin function; unfolded protein response.
© 2018 Federation of European Biochemical Societies.
Conflict of interest statement
Disclosure statement
The authors state no conflict of interest.
Figures
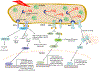

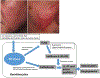
Similar articles
-
Misfolded proteins bind and activate death receptor 5 to trigger apoptosis during unresolved endoplasmic reticulum stress.Elife. 2020 Jan 6;9:e52291. doi: 10.7554/eLife.52291. Elife. 2020. PMID: 31904339 Free PMC article.
-
Adenoviral CCN gene transfers induce in vitro and in vivo endoplasmic reticulum stress and unfolded protein response.Biochim Biophys Acta. 2016 Nov;1863(11):2604-2612. doi: 10.1016/j.bbamcr.2016.07.006. Epub 2016 Jul 22. Biochim Biophys Acta. 2016. PMID: 27452908
-
Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer.Biochim Biophys Acta Rev Cancer. 2017 Jan;1867(1):58-66. doi: 10.1016/j.bbcan.2016.12.002. Epub 2016 Dec 15. Biochim Biophys Acta Rev Cancer. 2017. PMID: 27988298 Free PMC article. Review.
-
The role of endoplasmic reticulum stress in human pathology.Annu Rev Pathol. 2015;10:173-94. doi: 10.1146/annurev-pathol-012513-104649. Epub 2014 Oct 27. Annu Rev Pathol. 2015. PMID: 25387057 Free PMC article. Review.
-
The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease.Am J Physiol Cell Physiol. 2015 Mar 15;308(6):C415-25. doi: 10.1152/ajpcell.00334.2014. Epub 2014 Dec 24. Am J Physiol Cell Physiol. 2015. PMID: 25540175 Review.
Cited by
-
Patients with Darier disease have an increased risk of keratinocyte carcinoma: a Swedish registry-based nationwide cohort study.Orphanet J Rare Dis. 2024 Dec 16;19(1):463. doi: 10.1186/s13023-024-03497-z. Orphanet J Rare Dis. 2024. PMID: 39681873 Free PMC article.
-
DNAJB2 Attenuates Rosacea Skin Inflammation and Angiogenesis by Inhibiting the Endoplasmic Reticulum Stress-mediated TLR2/Myd88/NF-κB pathway.Inflammation. 2025 Mar 4. doi: 10.1007/s10753-025-02278-5. Online ahead of print. Inflammation. 2025. PMID: 40035989
-
scMINER: a mutual information-based framework for identifying hidden drivers from single-cell omics data.bioRxiv [Preprint]. 2023 Jan 27:2023.01.26.523391. doi: 10.1101/2023.01.26.523391. bioRxiv. 2023. Update in: Nat Commun. 2025 May 8;16(1):4305. doi: 10.1038/s41467-025-59620-6. PMID: 36747870 Free PMC article. Updated. Preprint.
-
Altered Serum Phospholipids in Atopic Dermatitis and Association with Clinical Status.JID Innov. 2021 Dec 22;2(2):100092. doi: 10.1016/j.xjidi.2021.100092. eCollection 2022 Mar. JID Innov. 2021. PMID: 35199091 Free PMC article.
-
Possible Role of lncRNA MEG3-microRNA-21 and Endoplasmic Reticulum (ER) Stress Proteins in the Pathogenesis of Psoriasis Vulgaris.Rep Biochem Mol Biol. 2022 Oct;11(3):367-376. doi: 10.52547/rbmb.11.3.367. Rep Biochem Mol Biol. 2022. PMID: 36718302 Free PMC article.
References
-
- Yoshida H (2007) ER stress and diseases, Febs j. 274, 630–58. - PubMed
-
- Sugiura K, Muro Y, Futamura K, Matsumoto K, Hashimoto N, Nishizawa Y, Nagasaka T, Saito H, Tomita Y & Usukura J (2009) The unfolded protein response is activated in differentiating epidermal keratinocytes, J Invest Dermatol. 129, 2126–35. - PubMed
-
- Common JE, O’Toole EA, Leigh IM, Thomas A, Griffiths WA, Venning V, Grabczynska S, Peris Z, Kansky A & Kelsell DP (2005) Clinical and genetic heterogeneity of erythrokeratoderma variabilis, J Invest Dermatol. 125, 920–7. - PubMed
-
- Frisoli ML & Harris JE (2017) Vitiligo: Mechanistic insights lead to novel treatments, J Allergy Clin Immunol. 140, 654–662. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

