Walking along chromosomes with super-resolution imaging, contact maps, and integrative modeling
- PMID: 30586358
- PMCID: PMC6324821
- DOI: 10.1371/journal.pgen.1007872
Walking along chromosomes with super-resolution imaging, contact maps, and integrative modeling
Abstract
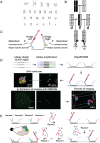
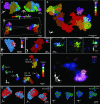
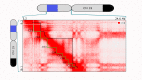
Chromosome organization is crucial for genome function. Here, we present a method for visualizing chromosomal DNA at super-resolution and then integrating Hi-C data to produce three-dimensional models of chromosome organization. Using the super-resolution microscopy methods of OligoSTORM and OligoDNA-PAINT, we trace 8 megabases of human chromosome 19, visualizing structures ranging in size from a few kilobases to over a megabase. Focusing on chromosomal regions that contribute to compartments, we discover distinct structures that, in spite of considerable variability, can predict whether such regions correspond to active (A-type) or inactive (B-type) compartments. Imaging through the depths of entire nuclei, we capture pairs of homologous regions in diploid cells, obtaining evidence that maternal and paternal homologous regions can be differentially organized. Finally, using restraint-based modeling to integrate imaging and Hi-C data, we implement a method-integrative modeling of genomic regions (IMGR)-to increase the genomic resolution of our traces to 10 kb.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: C-tW, BJB, RBM, SCN, SC, and HQN hold or have patent filings pertaining to Oligopaints and related technologies, including other oligo-based methods for imaging. These technologies may be licensed to ReadCoor, a company in which C-tW holds equity. PY and BJB have filed patents related to DNA-PAINT. DNA-PAINT technology has been licensed to Ultivue Inc., a company in which PY is an equity holder and cofounder. PY is also a co-founder of NuProbe Global.
Figures


References
-
- Cullen KE, Kladde MP, Seyfred MA. Interaction between transcription regulatory regions of prolactin chromatin. Science. 1993;261(5118):203–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

