A role for dopamine in the peripheral sensory processing of a gastropod mollusc
- PMID: 30586424
- PMCID: PMC6306152
- DOI: 10.1371/journal.pone.0208891
A role for dopamine in the peripheral sensory processing of a gastropod mollusc
Abstract
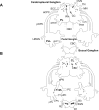
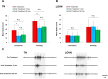
Histological evidence points to the presence of dopamine (DA) in the cephalic sensory organs of multiple gastropod molluscs, suggesting a possible sensory role for the neurotransmitter. We investigated the sensory function of DA in the nudipleuran Pleurobranchaea californica, in which the central neural correlates of sensation and foraging behavior have been well characterized. Tyrosine hydroxylase-like immunoreactivity (THli), a signature of the dopamine synthetic pathway, was similar to that found in two other opisthobranchs and two pulmonates previously studied: 1) relatively few (<100) THli neuronal somata were observed in the central ganglia, with those observed found in locations similar to those documented in the other snails but varying in number, and 2) the vast majority of THli somata were located in the peripheral nervous system, were associated with ciliated, putative primary sensory cells, and were highly concentrated in chemotactile sensory organs, giving rise to afferent axons projecting to the central nervous system. We extended these findings by observing that applying a selective D2/D3 receptor antagonist to the chemo- and mechanosensory oral veil-tentacle complex of behaving animals significantly delayed feeding behavior in response to an appetitive stimulus. A D1 blocker had no effect. Recordings of the two major cephalic sensory nerves, the tentacle and large oral veil nerves, in a deganglionated head preparation revealed a decrease of stimulus-evoked activity in the former nerve following application of the same D2/D3 antagonist. Broadly, our results implicate DA in sensation and engender speculation regarding the foraging-based decisions the neurotransmitter may serve in the nervous system of Pleurobranchaea and, by extension, other gastropods.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Sawin ER, Ranganathan R, Horvitz HR.C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

