Choosing an appropriate probiotic product for your patient: An evidence-based practical guide
- PMID: 30586435
- PMCID: PMC6306248
- DOI: 10.1371/journal.pone.0209205
Choosing an appropriate probiotic product for your patient: An evidence-based practical guide
Abstract
Introduction: Clinicians and patients face a daunting task when choosing the most appropriate probiotic for their specific needs. Available preparations encompass a diverse and continuously expanding product base, with most available products lacking evidence-based trials that support their use. Even when evidence exists, not all probiotic products are equally effective for all disease prevention or treatment indications. At this point in time, drug regulatory agencies offer limited assistance with regard to guidance and oversight in most countries, including the U.S.
Methods: We reviewed the current medical literature and sources on the internet to survey the types of available probiotic products and to determine which probiotics had evidence-based efficacy data. Standard medical databases from inception to June 2018 were searched and discussions with experts in the field were conducted. We graded the strength of the evidence for probiotics having multiple, randomized controlled trials and developed a guide for the practical selection of current probiotic products for specific uses.
Results: We found the efficacy of probiotic products is both strain-specific and disease-specific. Important factors involved in choosing the appropriate probiotic include matching the strain(s) with the targeted disease or condition, type of formulation, dose used and the source (manufacturing quality control and shelf-life). While we found many probiotic products lacked confirmatory trials, we found sufficient evidence for 22 different types of probiotics from 249 trials to be included. For example, several types of probiotics had strong evidence for the prevention of antibiotic-associated diarrhea [Saccharomyces boulardii I-745, a three-strain mixture (Lactobacillus acidophilus CL1285, L. casei Lbc80r, L. rhamnosus CLR2) and L. casei DN114001]. Strong evidence was also found for four types of probiotics for the prevention of a variety of other diseases/conditions (enteral-feed associated diarrhea, travellers' diarrhea, necrotizing enterocolits and side-effects associated with H. pylori treatments. The evidence was most robust for the treatment of pediatric acute diarrhea based on 59 trials (7 types of probiotics have strong efficacy), while an eight-strain multi-strain mixture showed strong efficacy for inflammatory bowel disease and two types of probiotics had strong efficacy for irritable bowel disease. Of the 22 types of probiotics reviewed, 15 (68%) had strong-moderate evidence for efficacy for at least one type of disease.
Conclusion: The choice of an appropriate probiotic is multi-factored, based on the mode and type of disease indication and the specific efficacy of probiotic strain(s), as well as product quality and formulation.
Trial registration: This review was registered with PROSPERO: CRD42018103979.
Conflict of interest statement
This study was funded by an unrestricted educational grant from Bio-K+ to cover publication fees. Bio-K Plus International, Inc. owns and manufactures the product Bio-K+ [“Lactic bacteria and their use in the prevention of diarrhea” US Patent no: 15/597,613]." JS and CT are on the Advisory Board of Bio-K+ (Canada). LVM is on the Advisory Board of Bio-K+ (Canada) and on the Biocodex Microbiome Advisory Board (France) and is a paid lecturer for Bio-K+, Biocodex and Lallemand. EJCG is on the following Advisory boards: Merck & Co, Bayer Pharmaceuticals, BioK+, Cutis Pharmaceuticals, Sanofi-Adventis, Summit Corp. plc, Kindred Healthcare Corp., Sankyo-Daichi, Paratek Pharma, Shionogi Inc.; and on the Speakers’ bureau for: Bayer Inc., Merck & Co, Medicines Co. Allergan Inc.; and also research grants with: Bayer Inc., Cutis Pharmaceuticals, Entasis Therapeutics,Merck & Co, Micromyx LLC, Parateck Pharmaceuticals, Spero Therapeutics, and Tetraphase Therapeutics. None of the authors own stock or equity in any of these companies. There are no further patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures
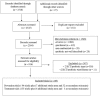
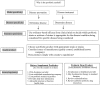
References
-
- Schultz M, Baranchi A, Thurston L, Yu YC, Wang L, Chen J, et al. Consumer demographics and expectations of probiotic therapy in New Zealand: results of a large telephone survey. N Z Med J. 2011; 124: 36–43. - PubMed
-
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. National health statistics reports; no 79. Hyattsville, MD: National Center for Health Statistics. 2015. Available at: https://nccih.nih.gov/research/statistics/NHIS/2012/natural-products/bio.... Accessed June 1, 2017. - PMC - PubMed
-
- Grand View Research. Nutraceuticals and Functional Foods. Global Industry Report. September 2016. Available at: http://www.grandviewresearch.com/industry-analysis/probiotics-market. Accessed June 1, 2017.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

