Rapid Colorimetric Detection of Bacterial Species through the Capture of Gold Nanoparticles by Chimeric Phages
- PMID: 30586498
- PMCID: PMC6396317
- DOI: 10.1021/acsnano.8b06395
Rapid Colorimetric Detection of Bacterial Species through the Capture of Gold Nanoparticles by Chimeric Phages
Abstract
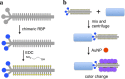
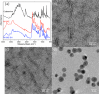
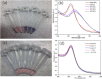
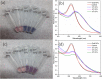
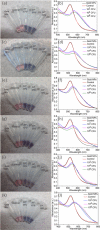
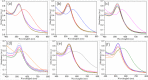
Rapid, inexpensive, and sensitive detection of bacterial pathogens is an important goal for several aspects of human health and safety. We present a simple strategy for detecting a variety of bacterial species based on the interaction between bacterial cells and the viruses that infect them (phages). We engineer phage M13 to display the receptor-binding protein from a phage that naturally targets the desired bacteria. Thiolation of the engineered phages allows the binding of gold nanoparticles, which aggregate on the phages and act as a signal amplifier, resulting in a visible color change due to alteration of surface plasmon resonance properties. We demonstrate the detection of two strains of Escherichia coli, the human pathogens Pseudomonas aeruginosa and Vibrio cholerae, and two strains of the plant pathogen Xanthomonas campestris. The assay can detect ∼100 cells with no cross-reactivity found among the Gram-negative bacterial species tested here. The assay can be performed in less than an hour and is robust to different media, including seawater and human serum. This strategy combines highly evolved biological materials with the optical properties of gold nanoparticles to achieve the simple, sensitive, and specific detection of bacterial species.
Keywords: bacteria; biosensor; detection; gold nanoparticles; phage display.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Controlled phage therapy by photothermal ablation of specific bacterial species using gold nanorods targeted by chimeric phages.Proc Natl Acad Sci U S A. 2020 Jan 28;117(4):1951-1961. doi: 10.1073/pnas.1913234117. Epub 2020 Jan 13. Proc Natl Acad Sci U S A. 2020. PMID: 31932441 Free PMC article.
-
Chimeric Phage Nanoparticles for Rapid Characterization of Bacterial Pathogens: Detection in Complex Biological Samples and Determination of Antibiotic Sensitivity.ACS Sens. 2020 May 22;5(5):1491-1499. doi: 10.1021/acssensors.0c00654. Epub 2020 May 5. ACS Sens. 2020. PMID: 32314570 Free PMC article.
-
A single thiolated-phage displayed nanobody-based biosensor for label-free detection of foodborne pathogen.J Hazard Mater. 2023 Feb 5;443(Pt A):130157. doi: 10.1016/j.jhazmat.2022.130157. Epub 2022 Oct 13. J Hazard Mater. 2023. PMID: 36265374
-
A colorimetric aptasensor based on gold nanoparticles for detection of microbial toxins: an alternative approach to conventional methods.Anal Bioanal Chem. 2022 Oct;414(24):7103-7122. doi: 10.1007/s00216-022-04227-9. Epub 2022 Jul 29. Anal Bioanal Chem. 2022. PMID: 35902394 Review.
-
Reporter Phage-Based Detection of Bacterial Pathogens: Design Guidelines and Recent Developments.Viruses. 2020 Aug 26;12(9):944. doi: 10.3390/v12090944. Viruses. 2020. PMID: 32858938 Free PMC article. Review.
Cited by
-
Potential of nanobiosensor in sustainable agriculture: the state-of-art.Heliyon. 2022 Dec 8;8(12):e12207. doi: 10.1016/j.heliyon.2022.e12207. eCollection 2022 Dec. Heliyon. 2022. PMID: 36578430 Free PMC article. Review.
-
Nanotechnology in Food and Plant Science: Challenges and Future Prospects.Plants (Basel). 2023 Jul 6;12(13):2565. doi: 10.3390/plants12132565. Plants (Basel). 2023. PMID: 37447126 Free PMC article. Review.
-
Colorimetric sensor array for the rapid distinction and detection of various antibiotic-resistant psychrophilic bacteria in raw milk based-on machine learning.Food Chem X. 2024 Mar 15;22:101281. doi: 10.1016/j.fochx.2024.101281. eCollection 2024 Jun 30. Food Chem X. 2024. PMID: 38544935 Free PMC article.
-
Rapid Detection of Lipopolysaccharide and Whole Cells of Francisella tularensis Based on Agglutination of Antibody-Coated Gold Nanoparticles and Colorimetric Registration.Micromachines (Basel). 2022 Dec 11;13(12):2194. doi: 10.3390/mi13122194. Micromachines (Basel). 2022. PMID: 36557493 Free PMC article.
-
Preparation of Bioconjugates of Chimeric M13 Phage and Gold Nanorods.Methods Mol Biol. 2024;2793:131-141. doi: 10.1007/978-1-0716-3798-2_9. Methods Mol Biol. 2024. PMID: 38526728 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

