Novel fluoronucleoside analog NCC inhibits lamivudine-resistant hepatitis B virus in a hepatocyte model
- PMID: 30586543
- PMCID: PMC9425639
- DOI: 10.1016/j.bjid.2018.11.005
Novel fluoronucleoside analog NCC inhibits lamivudine-resistant hepatitis B virus in a hepatocyte model
Abstract
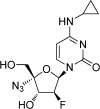
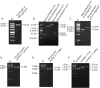
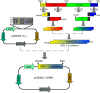
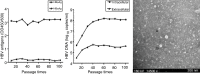
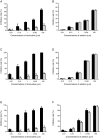
Antiviral drug resistance is the most important factor contributing to treatment failure using nucleos(t)ide analogs such as lamivudine for chronic infection with hepatitis B virus (HBV). Development of a system supporting efficient replication of clinically resistant HBV strains is imperative, and new antiviral drugs are needed urgently to prevent selection of drug-resistant HBV mutants. A novel fluorinated cytidine analog, NCC (N-cyclopropyl-4'-azido-2'-deoxy-2'-fluoro-β-d-cytidine), was recently shown to strongly inhibit human HBV in vitro and in vivo. This study was designed to evaluate the antiviral activity of NCC against lamivudine-resistant HBV. We generated a stable cell line encoding the major pattern of lamivudine-resistant mutations rtL180M/M204V and designated it "HepG2.RL1". Immuno-transmission electron microscopic examination and enzyme-linked immunosorbent assay were used to detect secretion of HBV-specific particles and antigens. Quantification of extracellular DNA and intracellular DNA of HepG2.RL1 cells by quantitative real-time polymerase chain reaction revealed >625-fold and >5556-fold increases in the 50% inhibitory concentration of lamivudine, respectively, compared with that for the wild-type virus. The results showed that NCC inhibited DNA replication and HBeAg production in wild-type or lamivudine-resistant HBV in a dose-dependent manner. In conclusion, screening for antiviral compounds active against lamivudine-resistant HBV can be carried out with relative ease using hepG2.RL1 cells. NCC is a potential antiviral agent against wild-type HBV and clinical lamivudine-resistant HBV and deserves evaluation for the treatment of HBV infection.
Keywords: HepG2.RL1 cells; Hepatitis B virus; Lamivudine-resistant; N-cyclopropyl-4′-azido-2′-deoxy-2′-fluoro-β-D- cytidine; rtL180M/M204V.
Copyright © 2018 Sociedade Brasileira de Infectologia. Published by Elsevier España, S.L.U. All rights reserved.
Figures
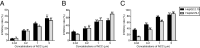
Similar articles
-
In vitro susceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir.Antivir Ther. 2004 Jun;9(3):353-63. Antivir Ther. 2004. PMID: 15259898
-
In vitro activity of cepharanthine hydrochloride against clinical wild-type and lamivudine-resistant hepatitis B virus isolates.Eur J Pharmacol. 2012 May 15;683(1-3):10-5. doi: 10.1016/j.ejphar.2012.02.030. Epub 2012 Feb 24. Eur J Pharmacol. 2012. PMID: 22387093 Free PMC article.
-
Generation of a human hepatoma cell line supporting efficient replication of a lamivudine resistant hepatitis B virus.J Virol Methods. 2014 Jun;201:51-6. doi: 10.1016/j.jviromet.2014.02.008. Epub 2014 Feb 28. J Virol Methods. 2014. PMID: 24583110
-
Evaluation of novel strategies to combat hepatitis B virus targetting wild-type and drug-resistant mutants in experimental models.Antivir Chem Chemother. 2001;12 Suppl 1:131-42. Antivir Chem Chemother. 2001. PMID: 11594680 Review.
-
Hepatitis B virus resistance to lamivudine and its clinical implications.Antivir Chem Chemother. 2002 May;13(3):143-55. doi: 10.1177/095632020201300301. Antivir Chem Chemother. 2002. PMID: 12448687 Review.
References
-
- Lu F., Zhuang H. Management of hepatitis B in China. Chin Med J. 2009;122:3. - PubMed
-
- Chemin I., Zoulim F. Hepatitis B virus induced hepatocellular carcinoma. Cancer Lett. 2009;286:52–59. - PubMed
-
- Lok A.S., McMahon B.J. Chronic hepatitis B. Hepatology. 2007;45:507–539. - PubMed
-
- Yuen M.F., Lai C.L. Treatment of chronic hepatitis B: evolution over two decades. J Gastroenterol Hepatol. 2011;26(Suppl. 1):138–143. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

