Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling
- PMID: 30586693
- PMCID: PMC6311723
- DOI: 10.1161/CIRCULATIONAHA.118.034588
Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling
Abstract
Background: Chronic kidney disease (CKD) increases cardiovascular risk. Underlying mechanisms, however, remain obscure. The uremic toxin indoxyl sulfate is an independent cardiovascular risk factor in CKD. We explored the potential impact of indoxyl sulfate on proinflammatory activation of macrophages and its underlying mechanisms.
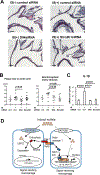
Methods: We examined in vitro the effects of clinically relevant concentrations of indoxyl sulfate on proinflammatory responses of macrophages and the roles of organic anion transporters and organic anion transporting polypeptides (OATPs). A systems approach, involving unbiased global proteomics, bioinformatics, and network analysis, then explored potential key pathways. To address the role of Delta-like 4 (Dll4) in indoxyl sulfate-induced macrophage activation and atherogenesis in CKD in vivo, we used 5/6 nephrectomy and Dll4 antibody in low-density lipoprotein receptor-deficient (Ldlr-/-) mice. To further determine the relative contribution of OATP2B1 or Dll4 to proinflammatory activation of macrophages and atherogenesis in vivo, we used siRNA delivered by macrophage-targeted lipid nanoparticles in mice.
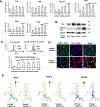
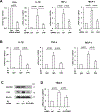
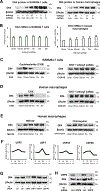
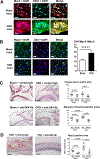
Results: We found that indoxyl sulfate-induced proinflammatory macrophage activation is mediated by its uptake through transporters, including OATP2B1, encoded by the SLCO2B1 gene. The global proteomics identified potential mechanisms, including Notch signaling and the ubiquitin-proteasome pathway, that mediate indoxyl sulfate-triggered proinflammatory macrophage activation. We chose the Notch pathway as an example of key candidates for validation of our target discovery platform and for further mechanistic studies. As predicted computationally, indoxyl sulfate triggered Notch signaling, which was preceded by the rapid induction of Dll4 protein. Dll4 induction may result from inhibition of the ubiquitin-proteasome pathway, via the deubiquitinating enzyme USP5. In mice, macrophage-targeted OATP2B1/Slco2b1 silencing and Dll4 antibody inhibited proinflammatory activation of peritoneal macrophages induced by indoxyl sulfate. In low-density lipoprotein receptor-deficient mice, Dll4 antibody abolished atherosclerotic lesion development accelerated in Ldlr-/- mice. Moreover, coadministration of indoxyl sulfate and OATP2B1/Slco2b1 or Dll4 siRNA encapsulated in macrophage-targeted lipid nanoparticles in Ldlr-/- mice suppressed lesion development.
Conclusions: These results suggest that novel crosstalk between OATP2B1 and Dll4-Notch signaling in macrophages mediates indoxyl sulfate-induced vascular inflammation in CKD.
Keywords: Notch signaling; atherosclerosis; chronic kidney diseases; indoxyl sulfate; inflammation; macrophage.
Figures



Comment in
-
Uremic Toxins Activate Macrophages.Circulation. 2019 Jan 2;139(1):97-100. doi: 10.1161/CIRCULATIONAHA.118.037308. Circulation. 2019. PMID: 30592654 Free PMC article. No abstract available.
References
-
- Tonelli M, Karumanchi SA, Thadhani R. Epidemiology and Mechanisms of Uremia-Related Cardiovascular Disease. Circulation. 2016;133:518–536. - PubMed
-
- Drueke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010;6:723–735. - PubMed
-
- Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jorres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work G. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

