Endothelial Hypoxia-Inducible Factor-2α Is Required for the Maintenance of Airway Microvasculature
- PMID: 30586708
- PMCID: PMC6340714
- DOI: 10.1161/CIRCULATIONAHA.118.036157
Endothelial Hypoxia-Inducible Factor-2α Is Required for the Maintenance of Airway Microvasculature
Abstract
Background: Hypoxia-inducible factors (HIFs), especially HIF-1α and HIF-2α, are key mediators of the adaptive response to hypoxic stress and play essential roles in maintaining lung homeostasis. Human and animal genetics studies confirm that abnormal HIF correlates with pulmonary vascular pathology and chronic lung diseases, but it remains unclear whether endothelial cell HIF production is essential for microvascular health. The large airway has an ideal circulatory bed for evaluating histological changes and physiology in genetically modified rodents.
Methods: The tracheal microvasculature of mice, with conditionally deleted or overexpressed HIF-1α or HIF-2α, was evaluated for anatomy, perfusion, and permeability. Angiogenic signaling studies assessed vascular changes attributable to dysregulated HIF expression. An orthotopic tracheal transplantation model further evaluated the contribution of individual HIF isoforms in airway endothelial cells.
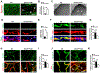
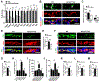
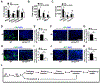
Results: The genetic deletion of Hif-2α but not Hif-1α caused tracheal endothelial cell apoptosis, diminished pericyte coverage, reduced vascular perfusion, defective barrier function, overlying epithelial abnormalities, and subepithelial fibrotic remodeling. HIF-2α promoted microvascular integrity in airways through endothelial angiopoietin-1/TIE2 signaling and Notch activity. In functional tracheal transplants, HIF-2α deficiency in airway donors accelerated graft microvascular loss, whereas HIF-2α or angiopoietin-1 overexpression prolonged transplant microvascular perfusion. Augmented endothelial HIF-2α in transplant donors promoted airway microvascular integrity and diminished alloimmune inflammation.
Conclusions: Our findings reveal that the constitutive expression of endothelial HIF-2α is required for airway microvascular health.
Keywords: HIF-2α; Notch; angiopoietin, TIE2; endothelial cells; hypoxia inducible factors; lung.
Figures





Similar articles
-
Tie2-dependent VHL knockdown promotes airway microvascular regeneration and attenuates invasive growth of Aspergillus fumigatus.J Mol Med (Berl). 2013 Sep;91(9):1081-93. doi: 10.1007/s00109-013-1063-8. J Mol Med (Berl). 2013. PMID: 23797537 Free PMC article.
-
Adenovirus-mediated HIF-1α gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection.J Clin Invest. 2011 Jun;121(6):2336-49. doi: 10.1172/JCI46192. Epub 2011 May 23. J Clin Invest. 2011. PMID: 21606594 Free PMC article.
-
Decreased lymphatic HIF-2α accentuates lymphatic remodeling in lymphedema.J Clin Invest. 2020 Oct 1;130(10):5562-5575. doi: 10.1172/JCI136164. J Clin Invest. 2020. PMID: 32673288 Free PMC article.
-
Multiplicity of hypoxia-inducible transcription factors and their connection to the circadian clock in the zebrafish.Physiol Biochem Zool. 2015 Mar-Apr;88(2):146-57. doi: 10.1086/679751. Epub 2015 Jan 14. Physiol Biochem Zool. 2015. PMID: 25730270 Review.
-
Effects of hypoxia-inducible factor-1α and hypoxia-inducible factor-2α overexpression on hepatocellular carcinoma survival: A systematic review with meta-analysis.J Gastroenterol Hepatol. 2021 Jun;36(6):1487-1496. doi: 10.1111/jgh.15395. Epub 2021 Jan 26. J Gastroenterol Hepatol. 2021. PMID: 33393670
Cited by
-
A paradoxical method to enhance compensatory lung growth: Utilizing a VEGF inhibitor.PLoS One. 2018 Dec 19;13(12):e0208579. doi: 10.1371/journal.pone.0208579. eCollection 2018. PLoS One. 2018. PMID: 30566445 Free PMC article.
-
Hypoxia-Inducible Factor Signaling in Inflammatory Lung Injury and Repair.Cells. 2022 Jan 6;11(2):183. doi: 10.3390/cells11020183. Cells. 2022. PMID: 35053299 Free PMC article. Review.
-
Sestrin2 serves as a scaffold protein to maintain cardiac energy and metabolic homeostasis during pathological stress.FASEB J. 2024 Oct 31;38(20):e70106. doi: 10.1096/fj.202401404R. FASEB J. 2024. PMID: 39404019 Free PMC article. Review.
-
A pleiotropic hypoxia-sensitive EPAS1 enhancer is disrupted by adaptive alleles in Tibetans.Sci Adv. 2022 Nov 25;8(47):eade1942. doi: 10.1126/sciadv.ade1942. Epub 2022 Nov 23. Sci Adv. 2022. PMID: 36417539 Free PMC article.
-
AZU1 (HBP/CAP37) and PRKCG (PKC-gamma) may be candidate genes affecting the severity of acute mountain sickness.BMC Med Genomics. 2023 Feb 20;16(1):28. doi: 10.1186/s12920-023-01457-3. BMC Med Genomics. 2023. PMID: 36803152 Free PMC article.
References
-
- Farkas L and Kolb M. Pulmonary microcirculation in interstitial lung disease. Proc Am Thorac Soc. 2011;8:516–521. - PubMed
-
- Dorfmuller P, Gunther S, Ghigna MR, Thomas de Montpreville V, Boulate D, Paul JF, Jais X, Decante B, Simonneau G, Dartevelle P, Humbert M, Fadel E and Mercier O. Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J. 2014;44:1275–1288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

