A Premature Termination Codon Mutation in MYBPC3 Causes Hypertrophic Cardiomyopathy via Chronic Activation of Nonsense-Mediated Decay
- PMID: 30586709
- PMCID: PMC6443405
- DOI: 10.1161/CIRCULATIONAHA.118.034624
A Premature Termination Codon Mutation in MYBPC3 Causes Hypertrophic Cardiomyopathy via Chronic Activation of Nonsense-Mediated Decay
Abstract
Background: Hypertrophic cardiomyopathy (HCM) is frequently caused by mutations in myosin-binding protein C3 ( MYBPC3) resulting in a premature termination codon (PTC). The underlying mechanisms of how PTC mutations in MYBPC3 lead to the onset and progression of HCM are poorly understood. This study's aim was to investigate the molecular mechanisms underlying the pathogenesis of HCM associated with MYBPC3 PTC mutations by utilizing human isogenic induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs).
Methods: Isogenic iPSC lines were generated from HCM patients harboring MYBPC3 PTC mutations (p.R943x; p.R1073P_Fsx4) using genome editing. Comprehensive phenotypic and transcriptome analyses were performed in the iPSC-CMs.
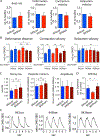
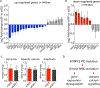
Results: We observed aberrant calcium handling properties with prolonged decay kinetics and elevated diastolic calcium levels in the absence of structural abnormalities or contracile dysfunction in HCM iPSC-CMs as compared to isogenic controls. The mRNA expression levels of MYBPC3 were significantly reduced in mutant iPSC-CMs, but the protein levels were comparable among isogenic iPSC-CMs, suggesting that haploinsufficiency of MYBPC3 does not contribute to the pathogenesis of HCM in vitro. Furthermore, truncated MYBPC3 peptides were not detected. At the molecular level, the nonsense-mediated decay pathway was activated, and a set of genes involved in major cardiac signaling pathways was dysregulated in HCM iPSC-CMs, indicating an HCM gene signature in vitro. Specific inhibition of the nonsense-mediated decay pathway in mutant iPSC-CMs resulted in reversal of the molecular phenotype and normalization of calcium-handling abnormalities.
Conclusions: iPSC-CMs carrying MYBPC3 PTC mutations displayed aberrant calcium signaling and molecular dysregulations in the absence of significant haploinsufficiency of MYBPC3 protein. Here we provided the first evidence of the direct connection between the chronically activated nonsense-mediated decay pathway and HCM disease development.
Keywords: cardiomyocytes; hypertrophic cardiomyopathy.
Figures



Comment in
-
Making Sense of Inhibiting Nonsense in Hypertrophic Cardiomyopathy.Circulation. 2019 Feb 5;139(6):812-814. doi: 10.1161/CIRCULATIONAHA.118.037936. Circulation. 2019. PMID: 30715935 No abstract available.
References
-
- Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic Cardiomyopathy: Present and future, with translation into contemporary cardiovascular medicine. JACC 2014;64:83–99. - PubMed
-
- Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy After 20 Years. JACC 2012;60:705–715. - PubMed
-
- Marston S, Copeland O, Jacques A, Livesey K, Tsang V, McKenna WJ, Jalilzadeh S, Carballo S, Redwood C, Watkins H. Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency. Circ Res 2009;105:219–222. - PubMed
-
- Vignier N, Schlossarek S, Fraysse B, Mearini G, Kramer E, Pointu H, Mougenot N, Guiard J, Reimer R, Hohenberg H, Schwartz K, Vernet M, Eschenhagen T, Carrier L. Nonsense-mediated mRNA decay and ubiquitin-proteasome system regulate cardiac myosin-binding protein C mutant levels in cardiomyopathic Mice. Circ Res 2009;105:239–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

