Dysfunctional and Proinflammatory Regulatory T-Lymphocytes Are Essential for Adverse Cardiac Remodeling in Ischemic Cardiomyopathy
- PMID: 30586716
- PMCID: PMC6322956
- DOI: 10.1161/CIRCULATIONAHA.118.036065
Dysfunctional and Proinflammatory Regulatory T-Lymphocytes Are Essential for Adverse Cardiac Remodeling in Ischemic Cardiomyopathy
Abstract
Background: Heart failure (HF) is a state of inappropriately sustained inflammation, suggesting the loss of normal immunosuppressive mechanisms. Regulatory T-lymphocytes (Tregs) are considered key suppressors of immune responses; however, their role in HF is unknown. We hypothesized that Tregs are dysfunctional in ischemic cardiomyopathy and HF, and they promote immune activation and left ventricular (LV) remodeling.
Methods: Adult male wild-type C57BL/6 mice, Foxp3-diphtheria toxin receptor transgenic mice, and tumor necrosis factor (TNF) α receptor-1 (TNFR1)-/- mice underwent nonreperfused myocardial infarction to induce HF or sham operation. LV remodeling was assessed by echocardiography as well as histological and molecular phenotyping. Alterations in Treg profile and function were examined by flow cytometry, immunostaining, and in vitro cell assays.
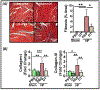
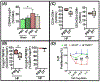
Results: Compared with wild-type sham mice, CD4+Foxp3+ Tregs in wild-type HF mice robustly expanded in the heart, circulation, spleen, and lymph nodes in a phasic manner after myocardial infarction, beyond the early phase of wound healing, and exhibited proinflammatory T helper 1-type features with interferon-γ, TNFα, and TNFR1 expression, loss of immunomodulatory capacity, heightened proliferation, and potentiated antiangiogenic and profibrotic properties. Selective Treg ablation in Foxp3-diphtheria toxin receptor mice with ischemic cardiomyopathy reversed LV remodeling and dysfunction, alleviating hypertrophy and fibrosis, while suppressing circulating CD4+ T cells and systemic inflammation and enhancing tissue neovascularization. Tregs reconstituted after ablation exhibited restoration of immunosuppressive capacity and normalized TNFR1 expression. Treg dysfunction was also tightly coupled to Treg-endothelial cell contact- and TNFR1-dependent inhibition of angiogenesis and the mobilization and tissue infiltration of CD34+Flk1+ circulating angiogenic cells in a C-C chemokine ligand 5/C-C chemokine receptor 5-dependent manner. Anti-CD25-mediated Treg depletion in wild-type mice imparted similar benefits on LV remodeling, circulating angiogenic cells, and tissue neovascularization.
Conclusions: Proinflammatory and antiangiogenic Tregs play an essential pathogenetic role in chronic ischemic HF to promote immune activation and pathological LV remodeling. The restoration of normal Treg function may be a viable approach to therapeutic immunomodulation in this disease.
Keywords: angiogenic cells; heart failure; inflammation; neovascularization; regulatory T cells.
Figures






Comment in
-
Protean Functions and Phenotypic Plasticity of Regulatory T Cells in Chronic Ischemic Heart Failure.Circulation. 2019 Jan 8;139(2):222-225. doi: 10.1161/CIRCULATIONAHA.118.036524. Circulation. 2019. PMID: 30615501 Free PMC article. No abstract available.
-
Response by Bansal et al to Letter Regarding Article, "Dysfunctional and Proinflammatory Regulatory T-Lymphocytes Are Essential for Adverse Cardiac Remodeling in Ischemic Cardiomyopathy".Circulation. 2019 Jun 11;139(24):e1035-e1036. doi: 10.1161/CIRCULATIONAHA.119.040737. Epub 2019 Jun 10. Circulation. 2019. PMID: 31180753 Free PMC article. No abstract available.
-
Letter by Wang et al Regarding Article, "Dysfunctional and Proinflammatory Regulatory T-Lymphocytes Are Essential for Adverse Cardiac Remodeling in Ischemic Cardiomyopathy".Circulation. 2019 Jun 11;139(24):e1033-e1034. doi: 10.1161/CIRCULATIONAHA.119.039990. Epub 2019 Jun 10. Circulation. 2019. PMID: 31180754 No abstract available.
References
-
- Dick SA and Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119:159–176. - PubMed
-
- Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK and Nahrendorf M. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res. 2016;119:853–864. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

