Senescent Phenotype Induced by p90RSK-NRF2 Signaling Sensitizes Monocytes and Macrophages to Oxidative Stress in HIV-Positive Individuals
- PMID: 30586719
- PMCID: PMC6957233
- DOI: 10.1161/CIRCULATIONAHA.118.036232
Senescent Phenotype Induced by p90RSK-NRF2 Signaling Sensitizes Monocytes and Macrophages to Oxidative Stress in HIV-Positive Individuals
Abstract
Background: The incidence of cardiovascular disease is higher in HIV-positive (HIV+) patients than it is in the average population, and combination antiretroviral therapy (cART) is a recognized risk factor for cardiovascular disease. However, the molecular mechanisms that link cART and cardiovascular disease are currently unknown. Our study explores the role of the activation of p90RSK, a reactive oxygen species-sensitive kinase, in engendering senescent phenotype in macrophages and accelerating atherogenesis in patients undergoing cART.
Methods: Peripheral whole blood from cART-treated HIV+ individuals and nontreated HIV-negative individuals was treated with H2O2 (200 µmol/L) for 4 minutes, and p90RSK activity in CD14+ monocytes was measured. Plaque formation in the carotids was also analyzed in these individuals. Macrophage senescence was determined by evaluating their efferocytotic ability, antioxidation-related molecule expression, telomere length, and inflammatory gene expression. The involvement of p90RSK-NRF2 signaling in cART-induced senescence was assessed by p90RSK-specific inhibitor (FMK-MEA) or dominant-negative p90RSK (DN-p90RSK) and NRF2 activator (NRF2A). Further, the severity of atherosclerosis was determined in myeloid cell-specific wild-type and DN-p90RSK transgenic mice.
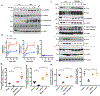
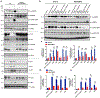
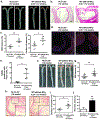
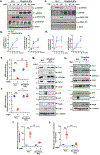
Results: Monocytes from HIV+ patients exhibited higher levels of p90RSK activity and were also more sensitive to reactive oxygen species than monocytes from HIV-negative individuals. A multiple linear regression analysis involving cART, Reynolds cardiovascular risk score, and basal p90RSK activity revealed that cART and basal p90RSK activity were the 2 significant determinants of plaque formation. Many of the antiretroviral drugs individually activated p90RSK, which simultaneously triggered all components of the macrophage senescent phenotype. cART inhibited antioxidant response element reporter activity via ERK5 S496 phosphorylation. NRF2A reversed the H2O2-induced overactivation of p90RSK in cART-treated macrophages by countering the induction of senescent phenotype. Last, the data obtained from our gain- or loss-of-function mice conclusively showed the crucial role of p90RSK in inducing senescent phenotype in macrophages and atherogenesis.
Conclusions: cART increased monocyte/macrophage sensitivity to reactive oxygen species- in HIV+ individuals by suppressing NRF2-ARE activity via p90RSK-mediated ERK5 S496 phosphorylation, which coordinately elicited senescent phenotypes and proinflammatory responses. As such, our report underscores the importance of p90RSK regulation in monocytes/macrophages as a viable biomarker and therapeutic target for preventing cardiovascular disease, especially in HIV+ patients treated with cART.
Keywords: HIV; antioxidants; atherosclerosis; reactive oxygen species; senescence; telomere.
Figures




Similar articles
-
An ERK5-NRF2 Axis Mediates Senescence-Associated Stemness and Atherosclerosis.Circ Res. 2023 Jun 23;133(1):25-44. doi: 10.1161/CIRCRESAHA.122.322017. Epub 2023 Jun 2. Circ Res. 2023. PMID: 37264926 Free PMC article.
-
Nucleus-mitochondria positive feedback loop formed by ERK5 S496 phosphorylation-mediated poly (ADP-ribose) polymerase activation provokes persistent pro-inflammatory senescent phenotype and accelerates coronary atherosclerosis after chemo-radiation.Redox Biol. 2021 Nov;47:102132. doi: 10.1016/j.redox.2021.102132. Epub 2021 Sep 20. Redox Biol. 2021. PMID: 34619528 Free PMC article.
-
A crucial role for p90RSK-mediated reduction of ERK5 transcriptional activity in endothelial dysfunction and atherosclerosis.Circulation. 2013 Jan 29;127(4):486-99. doi: 10.1161/CIRCULATIONAHA.112.116988. Epub 2012 Dec 14. Circulation. 2013. PMID: 23243209 Free PMC article.
-
How Monocytes Contribute to Increased Risk of Atherosclerosis in Virologically-Suppressed HIV-Positive Individuals Receiving Combination Antiretroviral Therapy.Front Immunol. 2019 Jun 19;10:1378. doi: 10.3389/fimmu.2019.01378. eCollection 2019. Front Immunol. 2019. PMID: 31275317 Free PMC article. Review.
-
The HIV Reservoir in Monocytes and Macrophages.Front Immunol. 2019 Jun 26;10:1435. doi: 10.3389/fimmu.2019.01435. eCollection 2019. Front Immunol. 2019. PMID: 31297114 Free PMC article. Review.
Cited by
-
Endothelial senescence-associated secretory phenotype (SASP) is regulated by Makorin-1 ubiquitin E3 ligase.Metabolism. 2019 Nov;100:153962. doi: 10.1016/j.metabol.2019.153962. Epub 2019 Aug 30. Metabolism. 2019. PMID: 31476350 Free PMC article.
-
Role of p90RSK in Kidney and Other Diseases.Int J Mol Sci. 2019 Feb 23;20(4):972. doi: 10.3390/ijms20040972. Int J Mol Sci. 2019. PMID: 30813401 Free PMC article. Review.
-
NRF2 signaling pathway and telomere length in aging and age-related diseases.Mol Cell Biochem. 2024 Oct;479(10):2597-2613. doi: 10.1007/s11010-023-04878-x. Epub 2023 Nov 2. Mol Cell Biochem. 2024. PMID: 37917279 Free PMC article. Review.
-
An ERK5-NRF2 Axis Mediates Senescence-Associated Stemness and Atherosclerosis.Circ Res. 2023 Jun 23;133(1):25-44. doi: 10.1161/CIRCRESAHA.122.322017. Epub 2023 Jun 2. Circ Res. 2023. PMID: 37264926 Free PMC article.
-
Aerobic Exercise Alters the Melanoma Microenvironment and Modulates ERK5 S496 Phosphorylation.Cancer Immunol Res. 2023 Sep 1;11(9):1168-1183. doi: 10.1158/2326-6066.CIR-22-0465. Cancer Immunol Res. 2023. PMID: 37307577 Free PMC article.
References
-
- van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F and study Anoc. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24:1527–1535. - PubMed
-
- Monsuez JJ, Belin C and Bouchaud O. Microvascular Function in Aging Among Women Living with HIV. Curr HIV/AIDS Rep. 2016;13:392–398. - PubMed
-
- Boccara F Cardiovascular health in an aging HIV population. AIDS. 2017;31 Suppl 2:S157–S163. - PubMed
-
- Kaplan-Lewis E, Aberg JA and Lee M. Atherosclerotic Cardiovascular Disease and Anti-Retroviral Therapy. Curr HIV/AIDS Rep. 2016;13:297–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL141106/HL/NHLBI NIH HHS/United States
- R01 HL149303/HL/NHLBI NIH HHS/United States
- R01 AG054328/AG/NIA NIH HHS/United States
- R01 HL130193/HL/NHLBI NIH HHS/United States
- R01 HL128155/HL/NHLBI NIH HHS/United States
- R01 HL123346/HL/NHLBI NIH HHS/United States
- R01 HL134740/HL/NHLBI NIH HHS/United States
- R01 NS066801/NS/NINDS NIH HHS/United States
- R01 MH099921/MH/NIMH NIH HHS/United States
- P30 AI078498/AI/NIAID NIH HHS/United States
- R01 HL118462/HL/NHLBI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 NS054578/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

