Glycemic Control, Cardiac Autoimmunity, and Long-Term Risk of Cardiovascular Disease in Type 1 Diabetes Mellitus
- PMID: 30586738
- PMCID: PMC6361693
- DOI: 10.1161/CIRCULATIONAHA.118.036068
Glycemic Control, Cardiac Autoimmunity, and Long-Term Risk of Cardiovascular Disease in Type 1 Diabetes Mellitus
Abstract
Background: Poor glycemic control is associated with increased risk of cardiovascular disease (CVD) in type 1 diabetes mellitus (T1DM); however, little is known about mechanisms specific to T1DM. In T1DM, myocardial injury can induce persistent cardiac autoimmunity. Chronic hyperglycemia causes myocardial injury, raising the possibility that hyperglycemia-induced cardiac autoimmunity could contribute to long-term CVD complications in T1DM.
Methods: We measured the prevalence and profiles of cardiac autoantibodies (AAbs) in longitudinal samples from the DCCT (Diabetes Control and Complications Trial) in participants with mean hemoglobin A1c (HbA1c) ≥9.0% (n=83) and ≤7.0% (n=83) during DCCT. We assessed subsequent coronary artery calcification (measured once during years 7-9 in the post-DCCT EDIC [Epidemiology of Diabetes Interventions and Complications] observational study), high-sensitivity C-reactive protein (measured during EDIC years 4-6), and CVD events (defined as nonfatal myocardial infarction, stroke, death resulting from CVD, heart failure, or coronary artery bypass graft) over a 26-year median follow-up. Cardiac AAbs were also measured in matched patients with type 2 diabetes mellitus with HbA1c ≥9.0% (n=70) and ≤7.0% (n=140) and, as a control for cardiac autoimmunity, patients with Chagas cardiomyopathy (n=51).
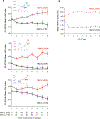
Results: Apart from HbA1c levels, the DCCT groups shared similar CVD risk factors at the beginning and end of DCCT. The DCCT HbA1c ≥9.0% group showed markedly higher cardiac AAb levels than the HbA1c ≤7.0% group during DCCT, with a progressive increase and decrease in AAb levels over time in the 2 groups, respectively ( P<0.001). In the HbA1c ≥9.0% group, 46%, 22%, and 11% tested positive for ≥1, ≥2, and ≥3 different cardiac AAb types, respectively, similar to patients with Chagas cardiomyopathy, compared with 2%, 1%, and 0% in the HbA1c ≤7.0% group. Glycemic control was not associated with AAb prevalence in type 2 diabetes mellitus. Positivity for ≥2 AAbs during DCCT was associated with increased risk of CVD events (4 of 6; hazard ratio, 16.1; 95% CI, 3.0-88.2) and, in multivariable analyses, with detectable coronary artery calcification (13 of 31; odds ratio, 60.1; 95% CI, 8.4-410.0). Patients with ≥2 AAbs subsequently also showed elevated high-sensitivity C-reactive protein levels (6.0 mg/L versus 1.4 mg/L in patients with ≤1 AAbs; P=0.003).
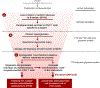
Conclusions: Poor glycemic control is associated with cardiac autoimmunity in T1DM. Furthermore, cardiac AAb positivity is associated with an increased risk of CVD decades later, suggesting a role for autoimmune mechanisms in the development of CVD in T1DM, possibly through inflammatory pathways.
Keywords: autoantibodies; biomarkers; cardiovascular diseases; diabetes mellitus; hyperglycemia.
Conflict of interest statement
Disclosures
The authors have no relevant disclosures.
Figures


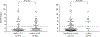
Comment in
-
Excess Cardiovascular Risk in Type 1 Diabetes Mellitus.Circulation. 2019 Feb 5;139(6):744-747. doi: 10.1161/CIRCULATIONAHA.118.038137. Circulation. 2019. PMID: 30715940 No abstract available.
Similar articles
-
The Association of Coronary Artery Calcification With Subsequent Incidence of Cardiovascular Disease in Type 1 Diabetes: The DCCT/EDIC Trials.JACC Cardiovasc Imaging. 2019 Jul;12(7 Pt 2):1341-1349. doi: 10.1016/j.jcmg.2019.01.014. Epub 2019 Mar 13. JACC Cardiovasc Imaging. 2019. PMID: 30878435 Free PMC article. Clinical Trial.
-
Effects of prior intensive versus conventional therapy and history of glycemia on cardiac function in type 1 diabetes in the DCCT/EDIC.Diabetes. 2013 Oct;62(10):3561-9. doi: 10.2337/db12-0546. Epub 2013 Mar 21. Diabetes. 2013. PMID: 23520132 Free PMC article. Clinical Trial.
-
Severe hypoglycemia and coronary artery calcification during the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study.Diabetes Res Clin Pract. 2015 Feb;107(2):280-9. doi: 10.1016/j.diabres.2014.10.007. Epub 2014 Oct 23. Diabetes Res Clin Pract. 2015. PMID: 25467622 Free PMC article.
-
Realising the long-term promise of insulin therapy: the DCCT/EDIC study.Diabetologia. 2021 May;64(5):1049-1058. doi: 10.1007/s00125-021-05397-4. Epub 2021 Feb 6. Diabetologia. 2021. PMID: 33550441 Review.
-
Neuropathy in the DCCT/EDIC-What Was Done Then and What We Would Do Better Now.Int Rev Neurobiol. 2016;127:9-25. doi: 10.1016/bs.irn.2016.02.020. Epub 2016 Apr 8. Int Rev Neurobiol. 2016. PMID: 27133142 Review.
Cited by
-
Commentary on risk factors for first and subsequent cardiovascular disease events in type 1 diabetes: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study.J Diabetes Investig. 2021 Mar;12(3):313-316. doi: 10.1111/jdi.13420. Epub 2020 Oct 28. J Diabetes Investig. 2021. PMID: 32997888 Free PMC article. No abstract available.
-
Screening for Subclinical Atherosclerosis and the Prediction of Cardiovascular Events in People with Type 1 Diabetes.J Clin Med. 2024 Feb 15;13(4):1097. doi: 10.3390/jcm13041097. J Clin Med. 2024. PMID: 38398409 Free PMC article. Review.
-
Understanding Competitive Endogenous RNA Network Mechanism in Type 1 Diabetes Mellitus Using Computational and Bioinformatics Approaches.Diabetes Metab Syndr Obes. 2021 Sep 8;14:3865-3945. doi: 10.2147/DMSO.S315488. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 34526791 Free PMC article.
-
Insulin's actions on vascular tissues: Physiological effects and pathophysiological contributions to vascular complications of diabetes.Mol Metab. 2021 Oct;52:101236. doi: 10.1016/j.molmet.2021.101236. Epub 2021 Apr 18. Mol Metab. 2021. PMID: 33878400 Free PMC article. Review.
-
Differential prognostic burden of cardiovascular disease and lower-limb amputation on the risk of all-cause death in people with long-standing type 1 diabetes.Cardiovasc Diabetol. 2022 May 9;21(1):71. doi: 10.1186/s12933-022-01487-8. Cardiovasc Diabetol. 2022. PMID: 35534880 Free PMC article.
References
-
- de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ, Zinman B and Eckel RH. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation 2014;130:1110–1130. doi: 10.1161/CIR.0000000000000034. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

