Endovascular Thrombus Removal for Acute Iliofemoral Deep Vein Thrombosis
- PMID: 30586751
- PMCID: PMC6389417
- DOI: 10.1161/CIRCULATIONAHA.118.037425
Endovascular Thrombus Removal for Acute Iliofemoral Deep Vein Thrombosis
Abstract
Background: The ATTRACT trial (Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis) previously reported that pharmacomechanical catheter-directed thrombolysis (PCDT) did not prevent postthrombotic syndrome (PTS) in patients with acute proximal deep vein thrombosis. In the current analysis, we examine the effect of PCDT in ATTRACT patients with iliofemoral deep vein thrombosis.
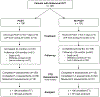
Methods: Within a large multicenter randomized trial, 391 patients with acute deep vein thrombosis involving the iliac or common femoral veins were randomized to PCDT with anticoagulation versus anticoagulation alone (No-PCDT) and were followed for 24 months to compare short-term and long-term outcomes.
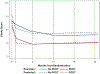
Results: Between 6 and 24 months, there was no difference in the occurrence of PTS (Villalta scale ≥5 or ulcer: 49% PCDT versus 51% No-PCDT; risk ratio, 0.95; 95% CI, 0.78-1.15; P=0.59). PCDT led to reduced PTS severity as shown by lower mean Villalta and Venous Clinical Severity Scores ( P<0.01 for comparisons at 6, 12, 18, and 24 months), and fewer patients with moderate-or-severe PTS (Villalta scale ≥10 or ulcer: 18% versus 28%; risk ratio, 0.65; 95% CI, 0.45-0.94; P=0.021) or severe PTS (Villalta scale ≥15 or ulcer: 8.7% versus 15%; risk ratio, 0.57; 95% CI, 0.32-1.01; P=0.048; and Venous Clinical Severity Score ≥8: 6.6% versus 14%; risk ratio, 0.46; 95% CI, 0.24-0.87; P=0.013). From baseline, PCDT led to greater reduction in leg pain and swelling ( P<0.01 for comparisons at 10 and 30 days) and greater improvement in venous disease-specific quality of life (Venous Insufficiency Epidemiological and Economic Study Quality of Life unit difference 5.6 through 24 months, P=0.029), but no difference in generic quality of life ( P>0.2 for comparisons of SF-36 mental and physical component summary scores through 24 months). In patients having PCDT versus No-PCDT, major bleeding within 10 days occurred in 1.5% versus 0.5% ( P=0.32), and recurrent venous thromboembolism over 24 months was observed in 13% versus 9.2% ( P=0.21).
Conclusions: In patients with acute iliofemoral deep vein thrombosis, PCDT did not influence the occurrence of PTS or recurrent venous thromboembolism. However, PCDT significantly reduced early leg symptoms and, over 24 months, reduced PTS severity scores, reduced the proportion of patients who developed moderate-or-severe PTS, and resulted in greater improvement in venous disease-specific quality of life.
Clinical trial registration: URL: https://www.clinicaltrials.gov . Unique identifier: NCT00790335.
Keywords: deep vein thrombosis; postthrombotic syndrome; thrombolysis, therapeutic.
Figures


Comment in
-
Reexamining the Open-Vein Hypothesis for Acute Deep Venous Thrombosis.Circulation. 2019 Feb 26;139(9):1174-1176. doi: 10.1161/CIRCULATIONAHA.118.037903. Circulation. 2019. PMID: 30802168 No abstract available.
-
"Early thrombus removal" in iliac-femoral deep vein thrombosis for prevention of post-thrombotic syndrome.Ann Transl Med. 2019 Dec;7(Suppl 8):S343. doi: 10.21037/atm.2019.09.102. Ann Transl Med. 2019. PMID: 32016061 Free PMC article. No abstract available.
-
Iliofemoral lysis for DVT remains ATTRACTive.Cardiovasc Diagn Ther. 2020 Apr;10(2):104-106. doi: 10.21037/cdt.2019.08.08. Cardiovasc Diagn Ther. 2020. PMID: 32420090 Free PMC article. No abstract available.
References
-
- Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011; 123:1788–1830. - PubMed
-
- Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, Janne D’Othee BM, Hofmann LV, Cardella JF, Kundu S, Lewis CA, Schwartzberg MS, Min RJ, Sacks D; Technology Assessment Committee of the Society of Interventional Radiology. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol 2006; 17:417–434. - PubMed
-
- O’Donnell TF, Browse NL, Burnand KG, Thomas ML. The socio-economic effects of an iliofemoral venous thrombosis. J Surg Res 1977; 22:483–488. - PubMed
-
- Comerota AJ, Gravett MH. Iliofemoral venous thrombosis. J Vasc Surg 2007; 46(5):1065–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

